|
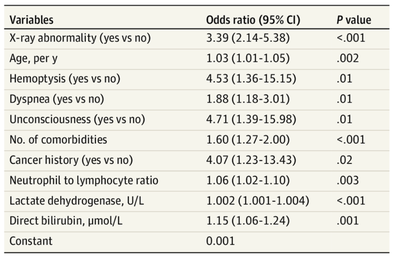
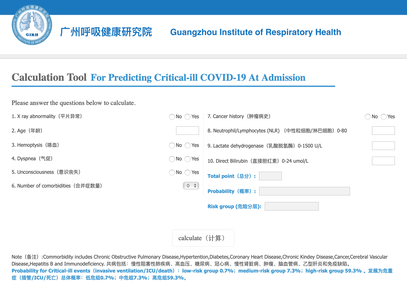
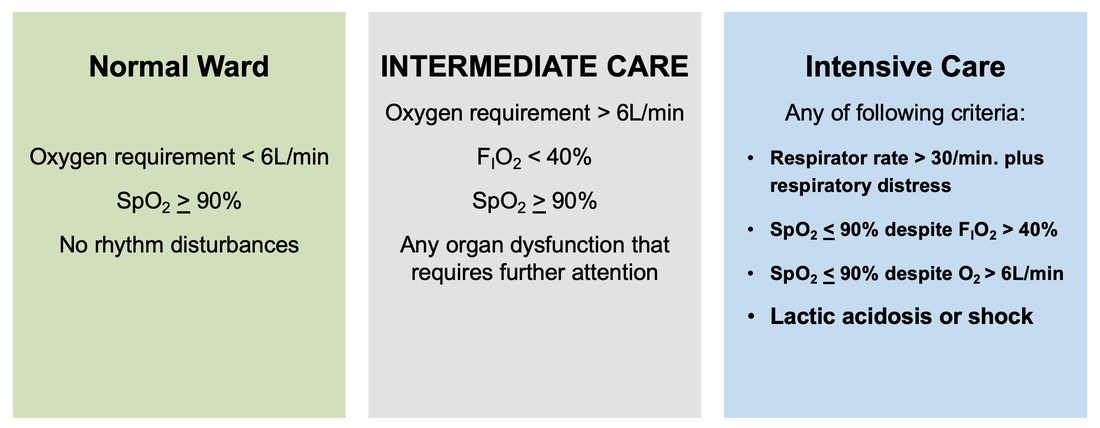
The treatment and management options of COVID-19 patient are rapidly evolving. The amount of research published daily is endless so that keeping an overview seems almost impossible. This short review of current publications is intended to overview current treatment options and its evidence. We will look at: - How do you Identify and Triage Patients at Risk for Severe Disease? - What about High Flow Nasal Cannulas (HFNC) and Non-Invasive Ventilation (NIV)? - Should we Prone Position the Spontaneously Breathing Patient? - When to Use Corticosteroids? - Should we Use Remdesivir? - What about Convalescent Plasma? - How do we Manage Thromboprophylaxis? How do you Identify and Triage Patients at Risk for Severe Disease? In an ideal world, we would be able to assess newly admitted patients with COVID-19 to predict the risk of getting critically ill in the course of the disease. Apart from a proper clinical assessment, JAMA published the COVID-GRAM Risk Score to address this problem. They used a cohort of 1590 patients to develop this score and validated this with a cohort of 710 patients. From 72 potential predictors, ten variables were independent predictive factors and were included in the risk score. The practicability in a clinical setting is not clear yet, and as any predictive score, there are several limitations when it comes to assessing a single patient instead of a cohort. The COVID-GRAM Score Calculator can be accessed via the following link: http://118.126.104.170/ Early identification of COVID-19 patients at risk for severe disease would be helpful for management. Every clinic/ ICU should have a triage and risk assessment tool at hand.
For triage, we use the following simple criteria: As a predictive assessment tool for severe disease the COVID-GRAM Calculator can be used:
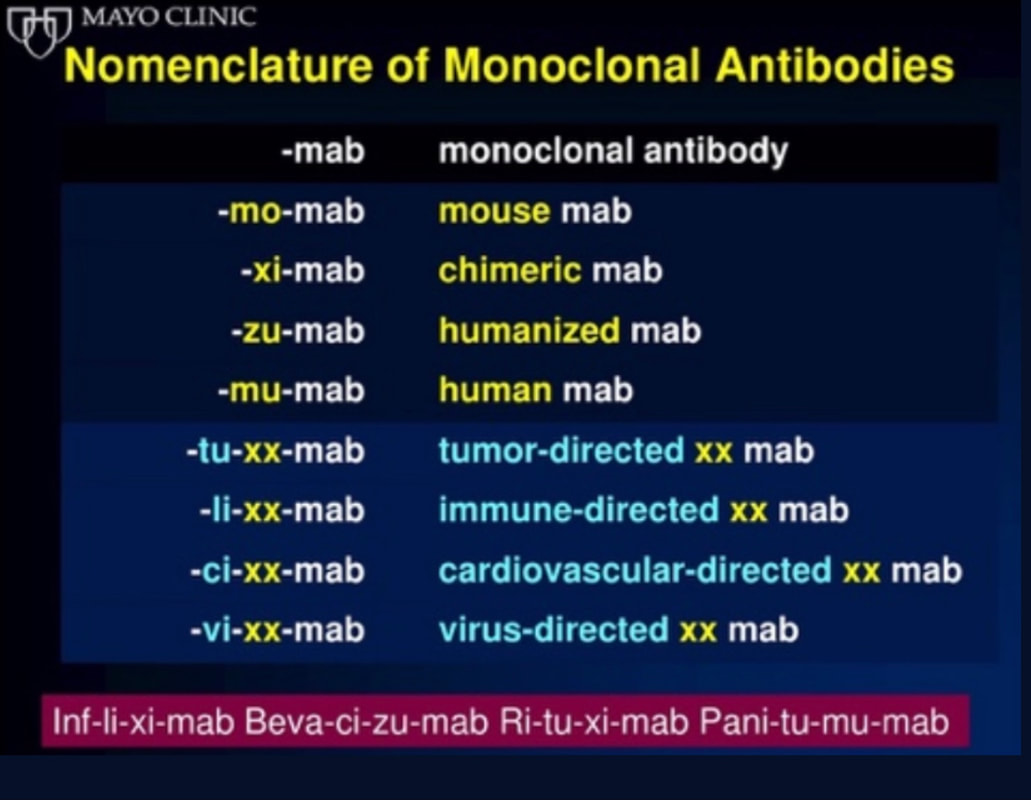
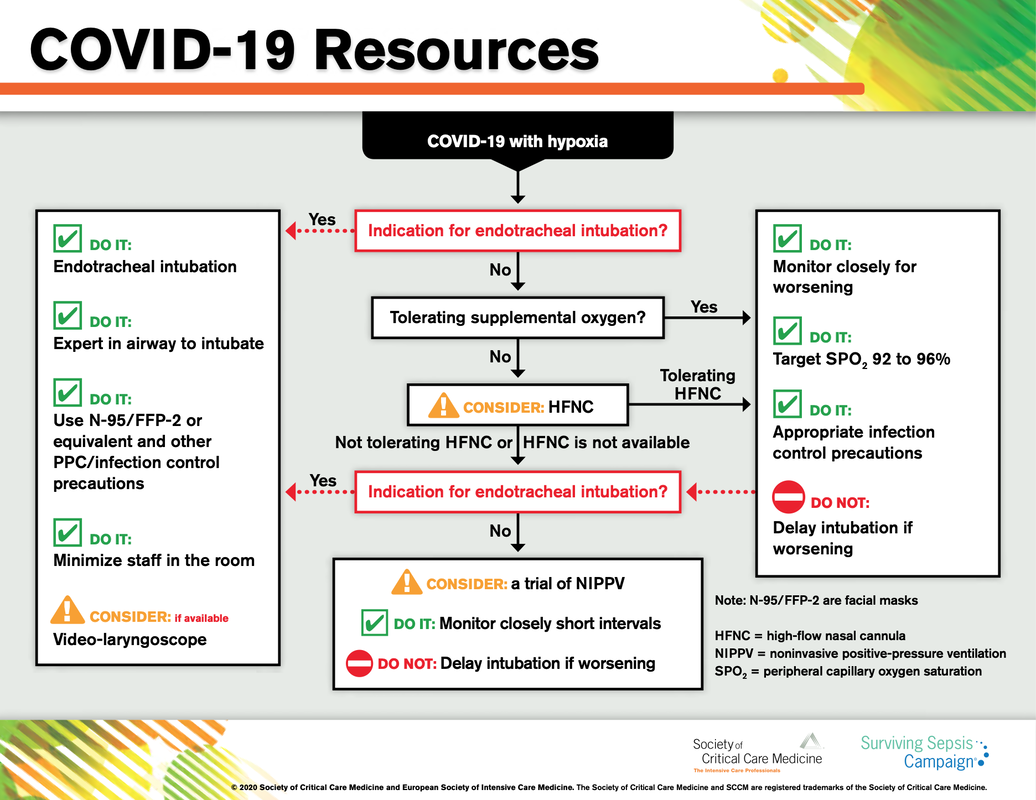
What about High Flow Nasal Cannulas (HFNC) and Non-Invasive Ventilation (NIV)? Especially at the beginning during the first wave of the pandemic, the use of HFNC and NIV was often avoided due to aerosolisation fear. Many ICU's tended to intubate their patients with respiratory failure relatively early. The lack of ventilators in some areas and reports that invasive ventilation is associated with high mortality (Zhou F, Lancet 2020; 395:1054) led to a constant change in management. KEEP IN MIND: Randomised-controlled studies for the treatment of COVID-19 patients with HFNC and NIV lack until now! Aerosolisation remains a big concern for health care workers (Niedermann MS; Am J Respir Crit Care Med 2020; 201:1019, Wu Z; JAMA 2020, February 24) and the amount of leakage flows is highly variable (Winck JC; Pulmonology 2020, April 20). Experience during the year 2020 showed, that most critical care providers have moved to use NIV and HFNC more frequently than initially. Proper personal protection equipment is essential and minimises risk for health care providers. Some evidence supports this approach (Avdeev SN, Am J Em Med AJEM Volume 39, p 154-157). NIV and HFNC is feasible in patients with COVID-19 and acute hypoxemic respiratory failure, even outside the ICU Helmet-NIV, leakage-free masks (non-vented masks) and double hose systems with virus-proof filters seem to be advantageous in this respect (Pfeiffer M; Pneumologie 2020, April 22). It is recommended that patients under HFNC should wear a surgical face mask over their cannulas Helmet NIV might advantageous compared to Mask NIV, though evidence is limited. (Patel BK et al. JAMA 2016. PMID: 27179847, single center study, trial stopped early, larger randomized-controlled studies awaited). KEEP IN MIND: Generally, there is only minimal evidence regarding the therapeutic benefit of these measures compared to their risks for the environment due to aerosolisation. Whether HFNC and NIV itself might produce self-inflicted lung injury (SILI) to some extend is not fully understood! Following patients should be considered for intubation and invasive ventilation - Severe hypoxemia (PaO2/FiO2 <150mmHg or respiratory rate >30/min) - Persistent or worsening respiratory failure (i.e. O2 sat <88%, RR > 36/min) - Neurologic deterioration - Intolerance of face mask or helmet - Airway bleeding - Copious respiratory secretions Should we Prone Position the Spontaneously Breathing Patient?Since the publication of Guerin C et al. (N Engl J Med 2013; 368:2159) prone positioning of patients with moderate to severe ARDS has become standard procedure in ICU's around the world. It is, therefore, evident that this treatment modality seems appropriate for COVID-19-induced lung injury, too. Trying to avoid intubations, clinicians rose the question, whether a prone position in the spontaneous breathing patient could avoid the need for invasive ventilation or even improve outcome. Ding L et al. (Crit Care 2020; 24:289) published a small multicenter study including 20 patients, whereas in 11 patients intubation could be avoided by prone positioning patients under NIV or HFNC. Telias et al. published an JAMA editorial (JAMA. 2020;323(22):2265-2267). He states that the prone position can improve oxygenation and can potentially result in less injurious ventilation. Unfortunately, this does not necessarily equate to lung protection and a better outcome. While improved oxygenation might prevent clinicians from intubating a patient, delayed intubation might worsen the patient's outcome. Regarding some evidence showing improved oxygenation during prone position, there are reasons to give it a try (Caputo ND et al. Acad Emerg Med Published online April 22, 2020). In the hypoxemic patient with no relevant respiratory distress awake prone positioning is a valid option - Use nasal cannulas or HFNC first - If comfortable enough, ask the patient to self-prone - Encourage the patient to remain in the prone position as long as well tolerated - Patients need close nursing and appropriate monitoring - Select prone positioning mattresses might be of help When to Use CorticosteroidsPatients with COVID-19 often show a biphasic course of the disease. The first phase is characterised by profound virus replication which decreases significantly after 5-7 days. After 7-10 days, a second phase develops in which an excessive or dysfunctional immune response can appear. This can lead to ARDS and multi-organ failure, which might be tackled by immunomodulating therapy. The largest, pragmatic randomised control trial we have at this stage is RECOVERY, performed in 176 hospitals around the UK and including more than 6400 patients (RECOVERY Collaborative group, N Engl J Med, July 17, 2020). COVID-19 patients that required oxygen or mechanical ventilation and presented with symptoms for at least seven days showed a significant reduction in 28-day mortality when treated with 6 mg Dexamethason OD for up to 10 days. Patients in the early viremic phase or patients that not required any oxygen performed worse with Dexamethasone. A broader insight into this topic brings a meta-analysis from JAMA in September 2020, including seven studies: DEXA-COVID19, CoDEX, RECOVERY, CAPE COVID, COVID STEROID, REMAP-CAP and Steroids-SARI. They ended up looking at 1703 patients and found a significant reduction in 28-day mortality when treated with steroids compared to placebo. Patients with COVID 19 that require oxygen, HFNC, NIV, mechanical ventilation or ECMO should be treated with steroids In patients not requiring oxygen, there is a trend towards harm when giving steroids - In these situations, steroids are NOT indicated Should we Use Remdesivir?Brief: Evidence in regards to the treatment with remdesivir is scattered and inconclusive. In the largest randomised control triad available so far is ACTT-1 looking at about 1600 patients (Beigel JH et al. N Engl J Med 2020; 383:1813-1826). In a few words, remdesivir showed a trend towards a 4-5 day shorter time to recovery, but not if symptoms existed for more than nine days. There was no significant influence on mortality, except maybe for patients requiring oxygen but not any help in ventilation. If at all, remdesivir might provide some advantage in a very selected patient group, but even this remains debatable. For this reason, many consider remdesivir the 'Tamiflu for COVID-19'. Two other papers remain to be mentioned briefly: Wang et al. (The Lancet; April 29) presented results from a relatively small study which was terminated early and showed no statistically significant clinical benefits of remdesivir - except for a trend towards a shorter duration of illness. Goldmann JD et al. presented the so-called '5 versus 10 days study', a phase 3 multicentre study with 397 patients. The primary outcome was their clinical status on day 14, secondary outcome patients with adverse events. Interestingly a 5-day course of remdesivir resulted in a better clinical outcome that a 10-day course. Again, It did not show any benefit compared to placebo. Remdesivir - The "Tamiflu for COVID-19" There is insufficient evidence to recommend the use of Remdesivir strongly. It is expensive, and if used, maybe there is only a short time window reasonable to act. Should We Use ECMO?During the early phase of the pandemic, first reports raised some concern that ECMO in COVID-19 patients might be associated with very high mortality (Henry BM et al. J Crit Care; 58:27). In the meanwhile, though we have new results from a more extensive cohort study looking at data from the Extracorporeal Life Support Organisation (ELSO, Barbaro RP et al. Lancet Volume 396, ISSUE 10257) The investigators looked at 1035 COVID-19 patients from 36 countries that were treated with ECMO (mean age 49 years, 74% male). 70% of all patients had relevant co-morbidities. The median time of ECMO support was 14 days. The incidence of in-hospital mortality 90 days after the initiation of ECMO was 37·4%. Mortality was 39% in patients with a final disposition of death or hospital discharge. These results are comparable with earlier mult-centre studies with patients suffering from non-COVID-19 ARDS (Combes A et al. N Engl J Med 2018; 378:1965). A retrospective cohort study from France looking at 83 patients treated with ECMO showed a probability to die after 60 days of 31%. Mortality at the time of the last follow-up was 36% (Schmidt et al. Lancet Respir Med 2020; 8:1121-1131). Various Societies recommend the use of ECMO in COVID-19 patients with treatment-refractory lung failure (Surviving Sepsis Campaign, ESICM, SCCCM and ELSO, WHO) Regarding the ongoing pandemic and limited resources, uniform indication and selection criteria for ECMO use should be available What about Convalescent Plasma?After a negative small randomised control trial (Li L et al. JAMA. 2020;324(5):460-470), a controversial Emergency Use Authorisation was granted by the FDA on 23.8.20 due to an observational study with a favourable effect on mortality with a high specific IgG content and onset less than days after symptom onset (Joyner MJ et al. MedRxiv; https://doi.org/10.1101/2020.08.12.20169359 - non peer-reviewed). At this stage the use of covalescent plasma can not be recommended How do we Manage Thromboprophylaxis?COVID 19 undoubtedly causes an inflammatory state that seems to trigger thrombotic activation in the venous and the arterial circulation. Thromboembolic complications are common, but the evidence is not robust on whether prophylactic or therapeutic doses should be used. Patients often have a significant elevation of D-dimers, an acute phase reactant representing the severity of disease rather than the dosage of thromboprophylaxis. One observational study looking at 1716 patients found no improved outcomes among in-hospital patients with COVID-19 when treated with therapeutic anticoagulation compared to prophylactic dosing. Moreover, patients who were started on anticoagulation for COVID-19 without evidence of thrombosis, new VTE, or new atrial fibrillation had worse outcomes compared to patients who were on prophylactic anticoagulation (Patel NG et al. Thrombosis Update; Volume 2, 2021, 100027) A case-based review of current literature and the COVID-19 specific coagulopathy end with the same finding that all in-hospital patients should receive prophylactic thromboprophylaxis. Whether a higher dose of prophylactic anticoagulation may be more effective is currently unknown (Chen EC et al. Oncologist. 2020 Oct; 25(10): e1500–e1508.). A small and retrospective study with 152 patients showed a lower risk of death and a lower cumulative incidence of thromboembolic events in patients with respiratory failure when a high-dose thromboprophylaxis was used. (Jonmarker S et al. Critical Care volume 24, Article number: 653 (2020)). Evidence supports the use of prophylactic thromboprophylaxis in patients with COVID-19 Whether a higher dose of anticoagulation might be more effective is currently unknown This single slide turn you into an expert in the nomenclature of monoclonal antibodies, but also helps to understand quickly what sort of medication your patient is treated with. Share and Care!
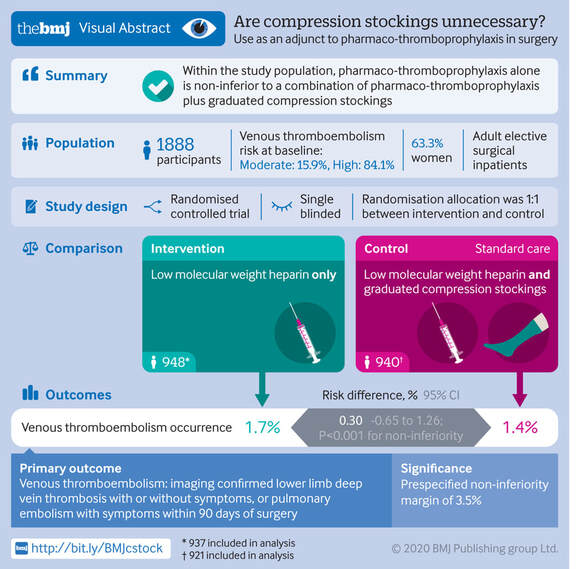
Mind the GAPS Study - Compression Stockings are Useless for Most Elective Surgery Patients!14/9/2020
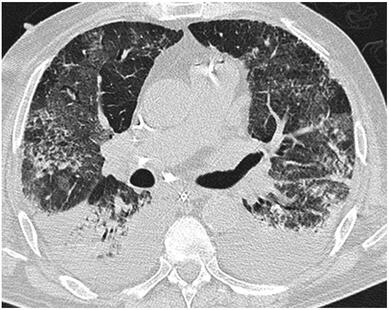
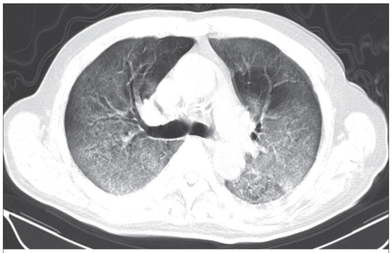
Cricoid pressure prevents aspirations, preoperative antibiotics avoid infections, and compression stockings protect against deep vein thrombosis. Many medical measures aim to reduce morbidity and mortality among patients, but unfortunately, the benefit of these measures is often not, or insufficiently, proven. Under certain circumstances, they may lead to additional problems or even cause harm (e.g. cricoid pressure Read Here). Time has definitely come to take a closer look at compression stockings for surgical patients. Apart from the fact that they look terrible, they are just as uncomfortable to wear and even carry certain risks in patients with peripheral vascular disease, for example. The effectiveness of compression stockings in modern practice has been questioned, but robust evidence has been lacking. This seems to change, as the long-awaited GAPS-Trial has been published and now provides further evidence on what concern patients undergoing elective surgery. Among this population, adding compression stockings to pharmaco-thromboprophylaxis was non-superior compared to pharmaco-thromboprophylaxis alone (primary outcome). There was also no difference in the quality of life outcomes found (secondary outcome). There is now some robust evidence to omit compression stockings in surgical patients that receive pharmacological thromboprophylaxis. Shalhou J. et al. BMJ 2020;369:m1309 The W.H.O. has repeatedly warned that antibiotic resistance is one of the biggest threats to global health today. Among all measures we can take to try and reduce this problem, merely avoiding unnecessary treatments is maybe one of the most effective. It is therefore pleasing that another piece of good evidence has been published, supporting the avoidance of antibiotics in the event of non-complicated diverticulitis (defined as non-perforated diverticulitis with a Hinchey 1a grade in computed tomography). The investigators performed a randomized, placebo-controlled, double-blind trial in which they compared 180 patients with non-complicated diverticulitis to receive either cefuroxime, metronidazole, and amoxicillin/clavulanic acid or placebo. They found No significant difference in the median time of hospital stay (primary outcome). Also, there were no significant differences between groups in adverse events, readmission to the hospital within one week, and readmission to the hospital within 30 days. These findings complement other studies indicating that observational treatment without antibiotics can be considered appropriate in patients with uncomplicated diverticulitis. The lastest updated surviving sepsis guidelines for COVID-19 patient recommends a high-peep strategy in the intubated, mechanically ventilated patient. As most of these patients present with moderate to severe ARDS, PEEP is used to keep lung areas open and therefor to improve oxygenation. This seems to be especially true in the classical case of ARDS, where the lung become 'wet' and 'heavy' which results in widespread atelectasis of the dependent parts of the lungs, often further complicated by pleural effusions. Classical CT appearance in the acute phase of ARDS is an opacification with an antero-posterior density gradient. Dense consolidation in the most dependent regions merges into a background of widespread ground-glass attenuation and the normal or hyperexpanded lung in the non-dependent areas (Howling SJ et al. Clin Radiol 1998;53(2):105-109). The theory behind these changes is that the increased weight of overlying lung causes compression-atelectasis posteriorly. The fact that prone positioning these patients quickly redistributes these gradients supports this theory (Desai SR et al. Anaesthesiology 1991;74(1):15-23). Chest CT's in patients with COVID-19 often show ground-glass opacification with or without consolidations. These are changes often seen in viral pneumonia. Several case series suggest, that CT abnormalities seem to be mostly bilateral and tend to have a peripheral distribution, often involving the lower lobes. In contrast to the classical ARDS pleural thickening, pleural effusion and lymphadenopathy seem to be a less common finding (Shi H et al. Lancet Infect Dis 2020). The leading problem in COVID-19 patients with ARDS is hypoxemia, while hypercapnia does not seem to be a significant problem. Sometimes profound hypoxemia does not seem to correlate with patient symptoms at all. In regards to the images above, atelectasis might not be the predominant reason for V/Q mismatches in these patients. Observations of mechanically ventilated patients in our unit and other hospitals in Switzerland have shown, that higher PEEP levels (15cmH2O and higher) often result in significantly reduced compliance values complicating ventilation and favouring the development of pulmonary over-inflation. This observation might support the theory that patients with COVID do not represent the traditional manner of ARDS with distinctive atelectasis. Another observation that supports this theory is that COVID-19 patients often do not respond as clearly to Prone Positioning as classical ARDS patients do. More probably, V/Q mismatch seems so happen on a more microscopical level in COVID-Patients. Lung compliance is often normal on these patients and, therefore, applying high PEEP-levels does NOT add any benefit at all. Maybe the principle of less is more also applies to COVID-19 patients we treat (Gattinoni L et al. Intensive Care Medicine; 46, pages780–782(2020)) Looking at the New Surviving Sepsis Campain COVID-19 Guidelines: Given these considerations, the strategy with High PEEP-levels in general should be questioned in principle. The European Society of Intensive Care Medicine ESICM and the Society of Critical Care Medicine SCCM have been very efficient in providing us health care workers with a guideline manuscript giving recommendations on the treatment of COVID-19 patients in a critical care setting. It is imperative to keep in mind that research is moving forward very quickly in these times and changes to these recommendations are likely to occur. A collection of many reliable OPEN ACCESS platforms on SARS-CoV-2 can be found on www.foam.education. Infection ControlWhen performing aerosol-generating procedures on patients with COVID-19 in the ICU, fitted respirator masks (N95 respirators, FFP2) should be used (in combination with full Personal Protective Equipement PPE) Aerosol-generating procedures on ICU patients with COVID-19 should be performed in a negative pressure room During usual care for non-ventilated and non-aerosol-generating procedures on mechanically ventilated (closed circuit) patients surgical masks are adequate For endotracheal intubation video-guided laryngoscopy should be used, if available In intubated and mechanically ventilated patients, endotracheal aspirates should be used for diagnostic testing Supportive CareIn COVID-19 patients with shock, dynamic parameters like skin temperature, capillary refilling time, and/or serum lactate measurement should be used in order to assess fluid responsiveness For the acute resuscitation of adults with COVID-19, a conservative over a liberal fluid strategy is recommended For the acute resuscitation of adults cristalloids should be used - avoid colloids! Buffered/balanced crystalloids should be used over unbalanced crystalloids Do NOT use hydroxyethyl starches! Do NOT use gelatins! Do NOT use dextrans! Avoid the routine use of albumin for initial resuscitation! In shock use norepinephrine/ noradrenaline as the first-line vasoactive agent The use of dopamine is NOT recommended Add vasopressin, if target MAP cannot be reached Titrate vasoactive agents to target a MAP of 60-65 mmHg, rather than higher MAP targets For patients in shock and with evidence of cardiac dysfunction and persistent hypoperfusion despite fluid resuscitation and norepinephrine, adding dobutamine should be used For persistent shock despite all these measures, low-dose corticosteroids should be tried Ventilatory SupportKeep peripheral saturation SpO2 above 90% with supplemental oxygen
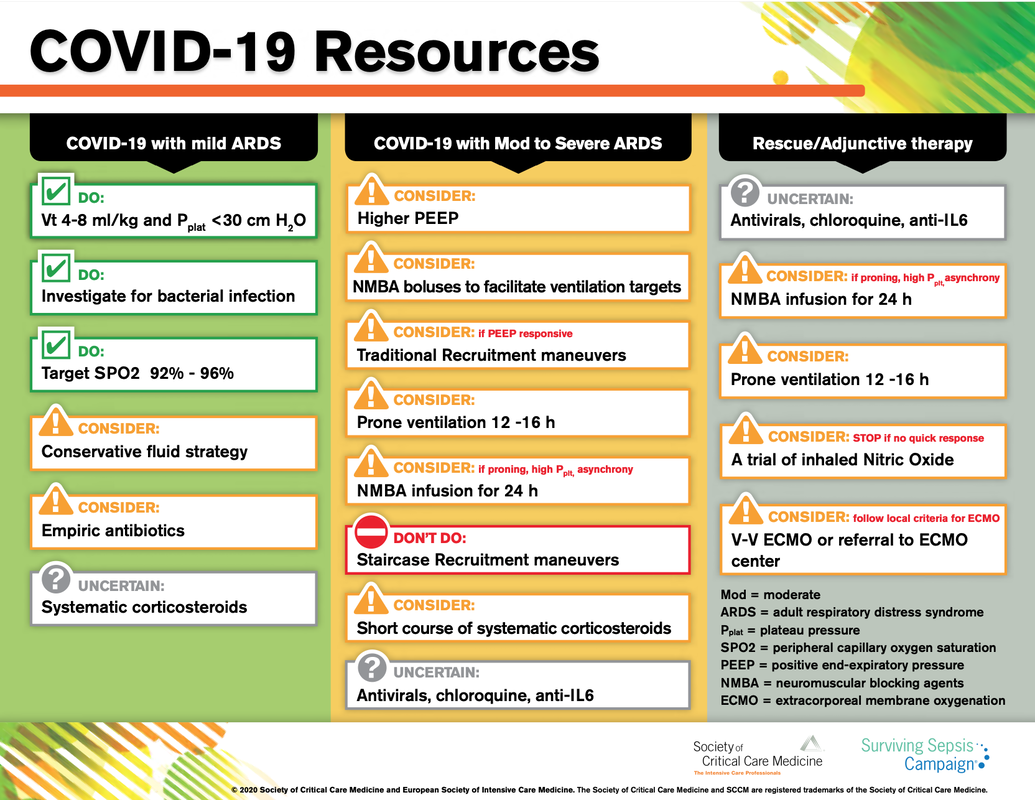
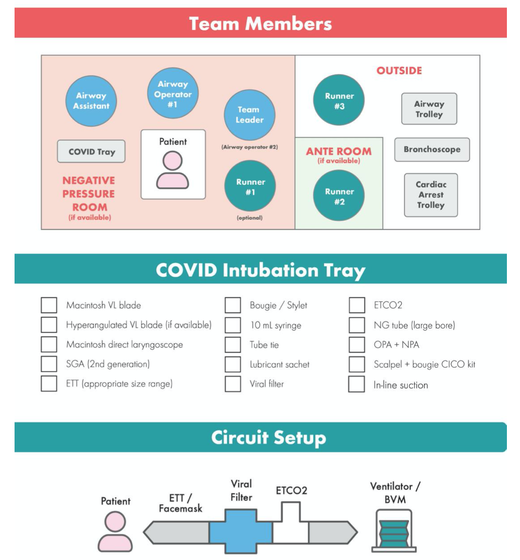
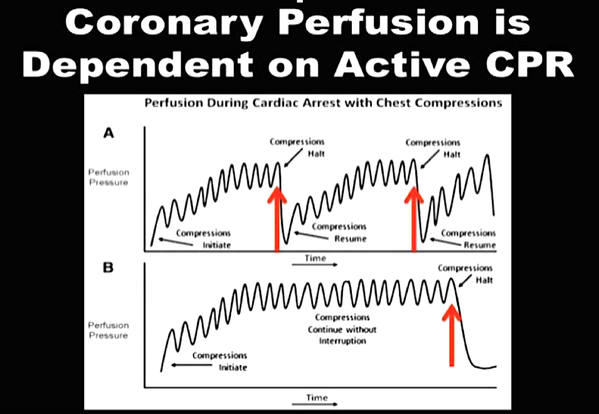
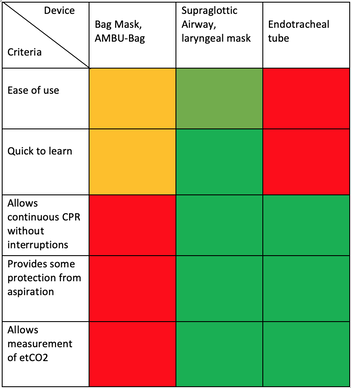
There is NO need for supplemental oxygen with SpO2 above 96% In acute hypoxemic respiratory failure despite conventional oxygen therapy, high-flow nasal cannulas (HFNC or High-Flow) should be used next High-Flow should be used over non-invasive ventilation (NIV) If High-Flow is not available and there is no urgent need for endotracheal intubation, NIV with close monitoring can be tried In the event of worsening respiratory status, early endotracheal intubation should be performed In mechanically ventilated patients, low-tidal volume ventilation should be used: 4 to 8 ml/kg In mechanically ventilated patients with ARDS targeting plateau pressures (Pplat) of < 30 cm H2O should be aimed for In patients with moderate to severe ARDS, a high-PEEP strategy should be used (PEEP >10cmH2O). Patients have to be monitored for potential barotrauma NOTE by Crit.Cloud: The strategy for high PEEP levels in general is currently discussed controversially. Observations in our own unit showed, that high PEEP levels tend to impaire compliance and therefor the quality of ventilation. Read also: "Less is More" in mechanical ventilatio, Gattinoni L. et al. Intensive Care Med (2020) 46:780-782 Patients with ARDS should receive a conservative/restrictive fluid strategy In moderate to severe ARDS, prone positioning for 12-16 hours is recommended To facilitate lung protective ventilation in moderate to severe ARDS, intermittent boluses of neuromuscular blocking agents (NMBA) should be used first In the event of persistent ventilator dyssynchrony, the need for ongoing deep sedation, prone ventilation, or persistently high plateau pressures, a continuous NMBA infusion for up to 48 hours should be used next Do NOT use inhaled nitric oxide in COVID-19 patients with ARDS routinely In severe ARDS and hypoxemia despite optimising ventilation and other rescue strategies, a trial of inhaled pulmonary vasodilator as a rescue therapy can be considered; if no rapid improvement in oxygenation is observed, the treatment should be tapered off If hypoxemia persists despite optimising ventilation, recruitment manoeuvres should be applied If recruitment manoeuvres are used, DO NOT use staircase (incremental PEEP) recruitment manoeuvres If all these measures fail, the patient should be considered for venovenous ECMO COVID-19 TherapyIn mechanically ventilated patients WITHOUT ARDS, systemic corticosteroids should NOT be used routinely In contrast, mechanically ventilated patients WITH ARDS, the use of systemic corticosteroids is recommended Mechanically ventilated patients with respiratory failure should be treated with empiric antimicrobials/antibacterial agents Critically ill patients with fever should be treated with paracetamol (acetominophen) for temperature control In critically ill patients standard intravenous immunoglobulins (IVIG) should NOT be used routinely Also, the routine use of convalescent plasma is NOT recommended The routine use of lopinavir/ritonavir (Kaletra®) is NOT recommended Currently, there is insufficient evidence to issue a recommendation on the use of other antiviral agents in critically ill adults with COVID-19 Currently, there is insufficient evidence to issue a recommendation on the use of recombinant interferons (rIFNs); chloroquine or hydroxychloroquine; tocilizumab (humanised immunoglobulin) The Aerosol-Danger of SARS-Cov-2The outbreak of the SARS Coronavirus-2 (SARS-CoV-2) in China 2019 has within a short time spread around the globe and is just about to hit central Europe. Although about 80% of all confirmed cases develop a mild febrile illness, around 17% develop severe Corona viral disease (COVID-19) with findings of acute respiratory distress syndrome (ARDS), of which about 4% will require mechanical ventilation. Since this virus, which was previously unknown to humans, spread rapidly around the globe, a large number of patients requiring intensive medical care now arise within a very short time. The lungs are the organs most affected by COVID-19 because the virus accesses host cells via the enzyme ACE2, which is most abundant in type II alveolar cells of the lungs. This results in mainly type 1 respiratory failure, which often requires urgent tracheal intubation and mechanical ventilation. Due to viral shedding in the patient's lungs, COVID-19 spread mainly via droplets. Events like coughing, high flow nasal oxygen (High-Flow), intubation and more can cause aerosol generation, allowing these airborne particles to travel even further distances. Performing endotracheal intubation in these patients is, therefore, a high-risk procedure, and it is required to adhere to certain principles to avoid infection of health care providers. The Safe Airway Societies of Australia and New Zealand have published a consensus statement that describes the problem very well and provides practical tips based on the currently available evidence. 1. Non Invasive Ventilation (NIV) and High Flow Nasal Oxygen (High-Flow)Current evidence suggests that the failure rate of NIV in COVID-19 patients seems to be similarly high as observed among Influenza A patients. Failure in these patients resulted in higher mortality. In general, NIV is recommended to be avoided or at least used very cautiously! The utility of High-Flow in viral pandemics in unknown. There is some evidence suggesting a decreased need for tracheal intubation compared to conventional oxygen therapy. High Flow Nasal Oxygen is worth a try, although it has to be assumed, that this is aerosol-generating. High-Flow should only be used in (negative pressure) airborne isolation rooms, and staff should wear full personal protective equipment (PPE) including N95/P2 masks. NIV and High-Flow are NOT recommended for patients with severe respiratory failure or when it seems clear that invasive ventilation is inevitable!
|
||||||||||
| Pocket Cards Toxine und Antidots | |
| File Size: | 165 kb |
| File Type: | |

There have been clues that influenza vaccination might reduce morbidity after surviving critical illness and Christiansen et al. have looked exactly into this topic.
The investigators examined whether an influenza vaccination (flu shot) affects the 1-year risk of myocardial infarction, stroke, heart failure, pneumonia, and death among ICU survivors aged 65 and older.
Performed a nationwide population-based cohort study
They used the Danish Intensive Care Database
To evaluate a total 89'818 ICU survivors from 2005 until 2015
It is noteworthy that
Influenza vaccinated patients (these were 39% of all) were older, had more chronic diseases and used more prescription medications!
Their findings show that
1. Influenza vaccinated patients showed an 8% decreased risk of death and a 16% reduced risk of hospitalisation for stroke within one year
2. Cardiac surgery patients were the subgroup that profited most
3. Unfortunately, no significant association was found for the risk of hospitalisation for myocardial infarction, heart failure or pneumonia.
The flu shot saves lives! This is another strong hint, that the influenza vaccination is clearly of benefit to all adults aged 65 and older. This is especially true for ICU survivors!
Christiansen at al. Intensive Care Med 2019 Jul;45(7):957-967.
Also worth mentioning:
Not only influenza A but also Influenza B infection can pose a risk for severe secondary infection in previously healthy and younger persons.
Aebi et al. BMC Infect Dis 2010 Oct 27;10:308.

- Sedation decreases sympathetic activity, aggression and leads to a non-REM-like state, which of all sedatives comes closest to natural sleep. Cognitive functions are maintained, and patients usually remain arousable.
- Dexmedetomidine has a particular analgesic effect via modulation in the region of the posterior horn of the spinal cord. This has shown to reduce the use of opiates.
- By reducing cerebral catecholamines, dexmedetomidine exerts a neuroprotective effect.
- Interestingly, sedation with dexmedetomidine is not associated with significant respiratory depression.
These properties pointed to a wide range of applications in the intensive care unit:
- Sedation in patients with non-invasive ventilation
- Weaning of invasively ventilated patients
- Agitated delirium
- Treatment of various withdrawal syndromes
- Fiberoptic awake intubation in theatre conditions
Dexmedetomidine comes with its side effects, though. Most commonly bradycardia and hypotension are observed, making second and third-degree heart block a contraindication. Also, nausea and a dry mouth might be seen.
Interestingly, prolonged use might be associated with some extent of discontinuation syndrome similar to clonidine. This involves hypertension, tachycardia, nervousness etc.
What Evidence Do We Have So Far?
- Might reduce the duration of sedation in mechanically ventilated patients, JAMA. 2007 Dec 12;298(22):2644-53.
- Might improve performance in patients with sepsis in regards to delirium, coma-free days and maybe even survival, Crit Care. 2010;14(2):R38. PMC2887145.
- Seems to reduce delirium in ICU and the need for mechanical ventilation in critically ill patients, JAMA. 2009 Feb 4;301(5):489-99.
- Seems to allow earlier extubation in mechanically ventilated patients and makes them more alert to communicate pain, and
- Compared to propofol, dexmedetomidine was comparable in terms of duration of mechanical ventilation, length of stay in ICU and hospital and also the incidence of hypotension and bradycardia. JAMA. 2012 Mar 21;307(11):1151-60.
- Some further evidence indicates that dexmedetomidine might be helpful in the treatment of mechanically ventilated patients with agitated delirium, resulting in more ventilator-free days. JAMA. 2016 Apr 12;315(14):1460-8.
According to all this, the question arises, whether we should use dexmedetomidine early in ventilated, critically ill patients.
Precisely this question was now addressed by Shehabi et al., published in the NEJM
They performed an
International (8 countries, 74 ICU's), randomised controlled, unblinded trial
In which they evaluated
4000 ICU patients that were expected to need mechanical ventilation for at least 48 hours and required sedation for safety or comfort
They compared
Patients sedated with propofol, midazolam or other agents as prescribed by the treating physician with patients receiving dexmedetomidine as a continuous infusion
(if DEX alone was insufficient, other agents could be added! In fact, 64% of patients also received propofol, 3% midazolam and 7% received both)
They found
1. No difference in 90-day mortality (primary outcome) and
2. No difference in death after 180 days, institutional dependency at 180 days, mean cognitive decline and assessment of the quality of life. Also no difference in median days free from coma to day 28 and median ventilator-free days at day 28 (all secondary outcomes)
3. Dexmedetomidine was though associated with significantly more events of bradycardia, hypotension (no further info on the use of vasopressors) and asystoles (14 vs 2; 7 required mechanical resuscitation measures)
- DEX is an attractive sedative in certain situations (alcohol withdrawal, other forms of delirium, weaning process etc.), BUT
- DEX doesn't seem to provide any advantage in the sedation of mechanically ventilated patients in the ICU and
- Might be problematic due to adverse cardiovascular effects, especially in this group of patients
Shehabe et al. N Engl J Med 2019; 380:2506-2517
Did you Know?

Acute decompensated heart failure comes in many ways as remains a challenge for optimal treatment since various conditions can cause it, e.g. advanced chronic heart failure, cardiogenic- and septic-shock, cardiac- and non-cardiac surgery, etc. Among many other interventions, the infusion of positive inotropes is often used on one side and vasodilators on the other side to stabilize the situation and improve cardiac function. Despite all these measures, the re-admission rate and mortality remain a significant problem among these patients.
While Levosimendan is still under investigation in the U.S., it is used worldwide for the short-term treatment of acutely decompensated severe chronic heart failure, especially when other treatment options have failed. Some recommend the usage of this drug in a different setting like sepsis-related heart failure or coronary heart disease.
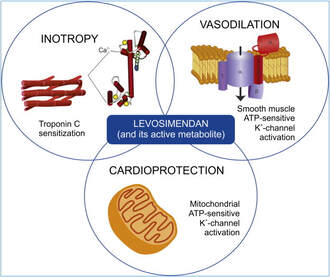
How does Levosimendan work?
Levosimendan has some remarkable properties, which are mainly caused by three mechanisms:
1. Enhancement of the calcium sensitivity of the myofilament by binding to troponin C.
2. Opening of adenosine triphosphate-sensitive potassium (KATP) channels in vasculature smooth muscle.
3. Opening of mitochondrial KATP channels.
These mechanisms result in a positive inotropy of the heart, an increase in stroke volume (SV) and therefore, cardiac output (CO). Besides, it's vasodilatory properties seems beneficial for coronary perfusion and reduce pulmonary capillary wedge pressure (PCWP).
All these effects do not appear to induce an unfavourable energy balance in the myocardial cell, and also the oxygen demand is not increased.
So what's the Evidence?
The Small Bits and Pieces
First publications back in the 90ies and early 2000s were indicative for the properties of levosimendan. Three studies were randomized and double-blinded but very low in patient numbers and therefore not powered enough to provide substantial evidence. Their statements were also not concerning patient outcome or mortality, but its results confirmed that levosimendan enhances cardiac output without oxygen wasting, is well tolerated and leads to favorable hemodynamic effects.
Lilleberg et al. Eur Heart J. 1998;19:660–668.
Ukkonen et al. Clin Pharmacol Ther. 2000;68:522–531.
Nieminen et al. J Am Coll Cardiol. 2000;36:1903–1912.
In 2003 Kivikko et al. published further data from another small trial indication that its beneficial effects (decreases in left and right heart filling pressures and in SVR, as well as increases in stroke volume and cardiac index) are maintained for at least 24 hours after discontinuation of a 24-hour infusion. At this point, it is worth mentioning that the author published these findings as a current employee of Orion Pharma, which manufactures levosimendan.
Kivikko et al. Circulation. 2003;107:81–86.
Further data indicated that its effect might be sustained for up to at least a week, although also this study included only 22 patients!
8. Lilleberg et al. Eur J Heart Fail. 2007;9:75–82.
A Lancet publication in 2002 by Follath et al. presented a multicentre, randomized, double-blind trial in which they compared the effect of levosimendan to dobutamine in patients that were admitted to a hospital with low-output heart failure and were judged to require hemodynamic monitoring and treatment with an intravenous inotropic agent. In these 203 patients, levosimendan had a consistently better effect than dobutamine on the individual hemodynamic variables at the end of the 24 h treatment period (cardiac output and pulmonary capillary wedge pressure). The change in clinical symptoms like fatigue and dyspnoea were not significantly different, though.
Interestingly, for the first time, the levosimendan group showed lower mortality than in the dobutamine group for up to 180 days. However, it's important to mention its absence of placebo control and its rather small sample size.
Follath et al. Lancet. 2002;360:196–202.
The Big Lumps
The SURVIVE Study
In the SURVIVE study Mebazaa et al. compared the efficacy and safety of intravenous levosimendan or dobutamine in patients hospitalized with acute decompensated heart failure who required inotropic support.
randomized, double-blind trial at 75 centers in 9 countries
in which they evaluated
1327 patients hospitalized with acute decompensated heart failure who required inotropic support
They found that
the addition of levosimendan to standard therapy resulted in fewer deaths compared with dobutamine, especially in the first few weeks after treatment
but
Levosimendan did not significantly reduce all-cause mortality at 180 days or affect any secondary clinical outcomes
The REVIVE Studies - Heart Failure
In the Randomized Multicenter Evaluation of Intravenous Levosimendan Efficacy studies (REVIVE I and REVIVE II) the efficacy of levosimendan on symptoms of heart failure during five days after starting a 24h trial drug infusion was assessed.
Each patient's clinical course over 5 days was determined by a composite of the patient's self-assessment of symptoms together with a physician's assessment of the occurrence of clinical deterioration.
The primary endpoint of the study was a new clinical composite endpoint (improvement, unchanged or worse - including death) derived from studies in chronic heart failure and first evaluated in 100 ADHF patients in the REVIVE I pilot study.
first large, prospective, randomized, double-blind, controlled trials
in which they evaluated
600 patients admitted at 103 sites in the United States, Australia, and Israel
with
worsening heart failure and dyspnea at rest despite treatment with intravenous diuretics, and left ventricular ejection fraction of < 35% measured within the last year
whereas
patients were randomized to either receive levosimendan for 24h or standard treatment alone
They found that after 5 days
- more patients receiving levosimendan experienced improvement compared with those who were on placebo (19.4% vs 14.6%, respectively, a 33% relative increase; P = .015)
- fewer patients receiving levosimendan worsened compared with patients who were on placebo (19.4% vs 27.2%, respectively), a 29% relative decrease, and
- fewer patients receiving levosimendan required rescue therapy (15.1%) vs placebo (26.2%), a 42% relative decrease.
Among other secondary endpoints, levosimendan improved
B-type natriuretic peptide (BNP) levels, length of hospital stay and dyspnoea at 6 hours
But
mortality at 90 days did not differ significantly between treatment arms (a secondary endpoint) and
the most common treatment-emergent cardiovascular adverse events were more frequent with levosimendan, including hypotension (50% vs 36%), ventricular tachycardia (25% vs 17%), and atrial fibrillation (8% vs 2%).
In this study investigators wanted to know whether in adult patients who have sepsis the application of levosimendan reduces the incidence and severity of acute organ dysfunction compared with placebo.
Randomised, double-blind, placebo-controlled multi-centre trial in 34 general ICUs in the UK
in which they evaluated
516 patients with suspected or confirmed infection and 2 or more SIRS criteria who were dependent on vasopressors for at least four hours to maintain their blood pressure.
They compared
the intravenous infusion of levosimendan or placebo for 24 hours in addition to standard care
and found:
- No significant difference in the daily Sequential Organ Failure Assessment (SOFA) score up to day 28 (primary outcome)
- No statistical difference on death at 28 days, at ICU discharge and hospital discharge (secondary outcome)
- And: The use of levosimendan was associated with more hemodynamic instability, lower mean arterial pressures, therefore more need for noradrenalin at 24h and significantly more supraventricular tachyarrhythmias (all secondary outcomes).
The CHEETAH Trial - Cardiac Surgery
The question in CHEETAH was if levosimendan compared to placebo reduces mortality in patients undergoing cardiac surgery with left ventricular dysfunktion.
This time Landoni et al. performed a
Multi-centre, randomised, placebo-controlledand parallel group designed trial
in which they evaluated
506 patients scheduled for cardiac surgery with peri-operative cardiovascular dysfunction
defined as
pre-operative left ventricular ejection fraction (LVEF) < 25%, pre-operative intra-aortic balloon pump (IABP), intra- or post-operative (within 24 hours) IABP or significant inotropic requirement
They compared
Levosimendan infusion continued for up to 48 hours (or until ICU discharge) or placebo
and found
No difference in mortality at thirty 30 days (primary outcome)
And no difference in survival over time, renal replacement therapy, the median duration of mechanical ventilation, the median hospital stay and interruptions due to adverse events (all secondary outcomes).
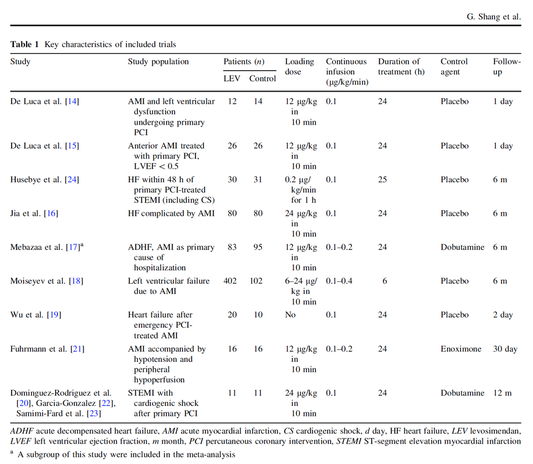
In contrast to these clinically somewhat discouraging results Shang et al. have published a meta-analysis in 2017 of randomized controlled trials looking at the usage of levosimendan in patients with heart failure, cardiogenic shock and acute coronary syndrome.
They ended up looking at a total of nine studies, most of them low in patient numbers and comparing levosimendan to either placebo or other drugs (dobutamine and enoximone).
According to these data the authors conclude that levosimendan is associated with reduced total mortality, decreased incidence of worsening HF, and improved hemodynamic outcomes and does not increase the risk of adverse events except for hypotension in patients with HF (including CS) complicating ACS. Thus, levosimendan should be recommended for routine clinical application in these patients.
Am J Cardiovasc Drugs 2017 Dec;17(6):453-463.
According to current evidence
- Levosimendan has interesting mechanisms of action and successfully seems to enhance cardiac output without additional oxygen wasting. This includes decreased right and left ventricular filling pressures, decreased SVR, as well as increases in stroke volume and cardiac index. Also, PCWP is decreased.
- In terms of various patient groups, patients with acute heart failure or acute on chronic heart failure appear to benefit in terms of clinical symptoms, if at all.
- Unfortunately, there is no convincing clinical evidence that levosimendan has any benefit in long term outcomes in terms of mortality.
- And, levosimendan seems to be associated with some potential treatment-emergent cardiovascular adverse events like hypotension, supraventricular and ventricular arrhythmia.
Search
Translate
Select your language above. Beware: Google Translate is often imprecise and might result in incorrect phrases!
Categories
All
Airway
Cardiovascular
Controversies
Endocrinology
Fluids
For A Smile ; )
Guidelines
Infections
Meducation
Neurology
Nutrition
Pharmacology
Procedures
Radiology
Renal
Respiratory
Resuscitation
SARS CoV 2
SARS-CoV-2
Sedation
Sepsis
Transfusion
Archives
January 2021
September 2020
March 2020
February 2020
January 2020
December 2019
November 2019
July 2019
May 2019
March 2019
February 2019
January 2019
December 2018
January 2018
October 2017
August 2017
June 2017
March 2017
February 2017
January 2017
October 2016
July 2016
June 2016
April 2016
February 2016
December 2015
October 2015
September 2015
August 2015
July 2015
June 2015
May 2015
April 2015
March 2015
January 2015
December 2014
November 2014
October 2014
September 2014
August 2014
July 2014
June 2014
May 2014
April 2014
March 2014
February 2014
January 2014
December 2013
November 2013
Author
Timothy Aebi























 RSS Feed
RSS Feed


