|
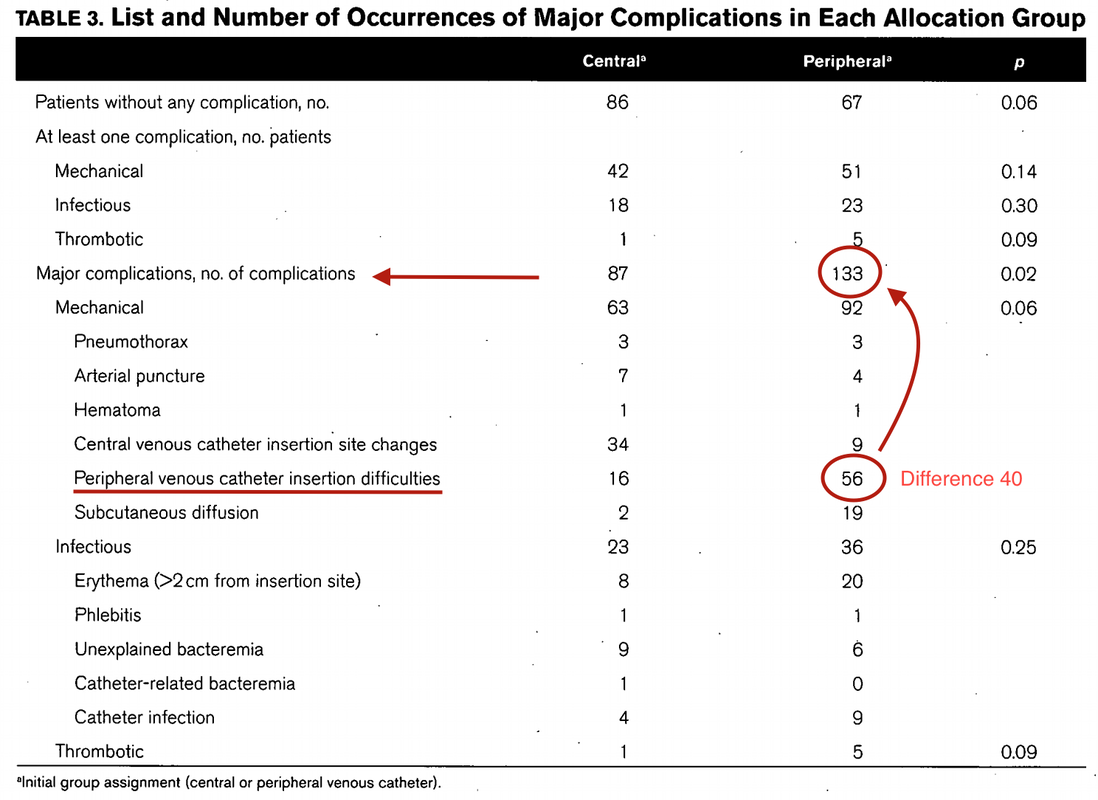
Teaching in medical school and opinions in literature are in agreement: The application of vasopressors requires central venous access. The reason for this are concerns that vasopressors given over a peripheral venous catheter (PCV) may cause phlebitis or even worse necrosis or ischemia through extravasation. While irritation of a peripheral vein is often observed with the administration of drugs like potassium or amiodarone, this usually is not the case with the application of, e.g. norepinephrine. Besides, it is essential to keep in mind that the insertion of a central venous catheter (CVC) is technically demanding and takes a certain amount of time when performed correctly. The procedure is also associated with potentially dangerous complications that might be hazardous to the patient. Therefore a fundamental question arises: Do all patients that require vasopressors need a central venous catheter? What about the peripheral access (PVC) - Any dangers there?
|
Search
|














 RSS Feed
RSS Feed


