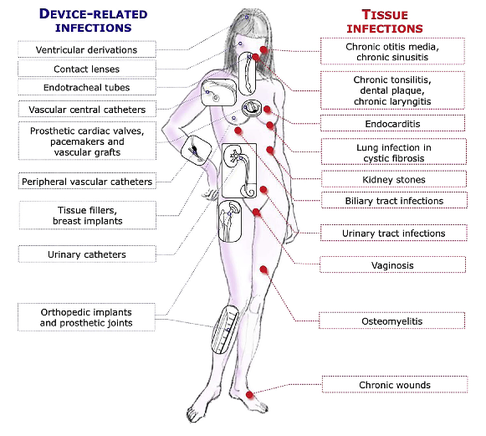
 The European Society for Clinical Microbiology and Infectious Diseases has now released new guidelines on the diagnosis and treatment of biofilm infections. Written for clinical microbiologists and infectious disease specialists this paper is a MUST READ for anyone involved in treating critically ill patients. These guidelines outline the nature and properties of biofilms and and their implications on mostly chronic infections caused. As biofilms are very common in critically ill patients it is important to know what specific problems you might encounter, how to proceed and perform a proper diagnosis and what are the essential bits and pieces in the prevention and treatment of biofilm infections. The article is OPEN ACCESS: Clin Microbiol Infect. 2015 Jan 14. pii: S1198-743X(14)00090-1.  In the late 1960's the technology of counter-pulsation by using an intra-aortic balloon pump (IABP) was introduced into clinical work. Based on the principle of diastolic inflation and systolic deflation, IABP counter-pulsation improves diastolic coronary artery blood flow and decreases left ventricular afterload. Up to the year 2009, 2012 respectively, the usage of an IABP in patients with ST-segment elevation myocardial infarction and cardiogenic shock was considered a class IC recommendation (reminder: levels of evidence). Since then a couple of well conducted, larger trials have failed to show a positive impact of IABP especially on mortality. In regards of the most recent meta-analysis in JAMA we provide a short overview of the most important publications. It's interesting to see that the balloons undermining started with a meta-analysis and for the the time being ends with one. Stitch no.1 The first notable hole in the ballon was caused by Sjauw et al.'s systematic review and meta-analysis in the European Heart Journal in 2009. Their pooled randomized data consisting of two separate meta-analyses did not support the use of an IABP in patients with high risk STEMI. They concluded that there is insufficient evidence endorsing the current guideline recommendation for the use of IABP therapy in the setting of STEMI complicated by cardiogenic shock. This publication was one of the main reasons for the expert panel of the European Society of Cardiology to change the recommendation (ESC Guidelines 2012) to use an IABP in patients with STEMI from IC to IIB. Stitch 2 and 3 In the same year 2012 Thiele et al. published their first IABP-SHOCK II results in the NEJM. Their randomized, prospective, open-label, multicenter trial showed no reduction in the 30-day mortality compared to the best available medical therapy alone in patients with myocardial infarction-induced cardiogenic shock and planned early revascularization (PCI or CABG). One year later the IABP-SHOCK II investigators published their final 12-months results in The Lancet. They came to the final conclusion that in patients undergoing early revascularization for myocardial infarction with cardiogenic shock, IABP did not reduce 12-month all-cause mortality. Stitch no. 4 In 2013 Ranucci at al. presented the results of their single-center prospective randomized controlled trial looking at the usage of a preoperative IABP in high-risk patients undergoing surgical coronary revascularization. By looking at a total of 110 patients with an ejection fraction below 35% and no hemodynamic instability there was no improvement in outcome when inserting an IABP preoperatively. Preliminary Final Stitch So finally Ahmad and his team decided to assess IABP efficacy in acute myocardial infarction by performing an updated meta-analysis. Main outcome was 30-day mortality. They included 12 eligible RCTs randomizing 2123 patients and found no improvement in mortality among patients with acute myocardial infarction... regardless of whether patients had cardiogenic shock or not! A look at another 15 eligible observational studies with a total of 15 530 patients showed basically conflicting results which was explained by the differences between studies in the balance of risk factors between IABP and non-IABP groups. It seems that the IABP fails to show its assumed efficacy in patients with myocardial infarction and cardiogenic shock, especially when early revascularization (PCI or CABG) is available. As a general consideration and also when no early revascularisation is available the use of another left-ventricular assist device like the Impella pump might prove to be a good and easy to use alternative (see blow). Sjauw KD et al. Eur Heart J 30: 459-468 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J 33: 2569-2619 OPEN ACCESS Thiele et al. N Engl J Med 2012; 367:1287-1296 OPEN ACCESS Thiele et al. The Lancet, Volume 382, Issue 9905, Pages 1638 – 1645 Crit Care Med. 2013 Nov;41(11):2476-83 JAMA Intern Med. Published online March 30, 2015 Short film on the principle of the Impella pump 2.5. Bare in mind that this device can actually be easily inserted in the environment of ICU and positioned by using transthoracic echo TTE.  Almost exactly one year ago the Cochrane Library published an intervention review on the prevention and treatment of influenza with neuraminidase inhibitors in adults and children. The reason for this review was the fact that many countries stockpile these drugs and the WHO classified them as an essential medicine. Jefferson et al. used the data of 46 trials with oseltamivir or zanamivir for this review. They basically conclude that: - Both drugs shorten the duration of symptoms of influenza-like symptoms by less than a day - Oseltamivir did not affect the number of hospitalizations - Prophylaxis trials showed a reduced risk of symptomatic influenza in individuals and households, but no definite conclusion can be made - Oseltamivir use was associated though with nausea, vomiting, headaches, renal and psychiatric events ...and finally write: 'The influenza virus-specific mechanism of action proposed by the producers does not fit the clinical evidence'. This review certainly undermined the importance of oseltamivir for many of us. The Cochrane review though did not look at outcomes like mortality, but the Lancet Respiratory Medicine did! Stella G at al. have now published a large systematic review which included 29'234 patients from 78 studies during the period from 2009 to 2014. Their findings come rather surprisingly: - Compared with no treatment, neuraminidase inhibitor treatment (irrespective of timing) was associated with a reduction in mortality risk - Compared with later treatment, early treatment (within 2 days of symptom onset) was associated with a reduction in mortality risk - The reduction in mortality risk was observed when treatment was started up to 5 days of symptoms onset There still seem to be some good reasons to use oseltamivir in critically ill patients with suspected or proven influenza... up to 5 days of symptoms onset! Jefferson T et al. The Cochrane Collaboration, Published Online: 10 APR 2014 The Cochrane Collaboration News Release 10 April 2014 Muthuri, Stella G et al. The Lancet Respiratory Medicine , Volume 2 , Issue 5 , 395 - 404 |
Search
|

 RSS Feed
RSS Feed


