|
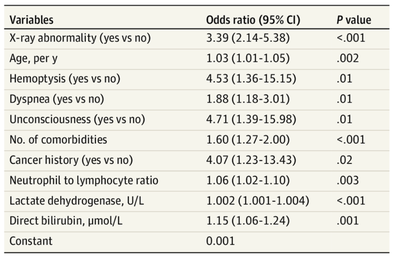
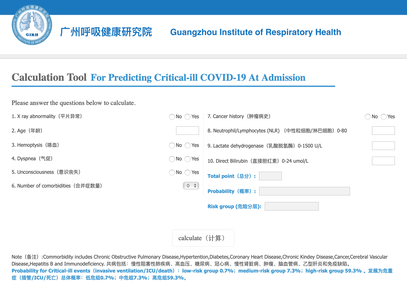
The treatment and management options of COVID-19 patient are rapidly evolving. The amount of research published daily is endless so that keeping an overview seems almost impossible. This short review of current publications is intended to overview current treatment options and its evidence. We will look at: - How do you Identify and Triage Patients at Risk for Severe Disease? - What about High Flow Nasal Cannulas (HFNC) and Non-Invasive Ventilation (NIV)? - Should we Prone Position the Spontaneously Breathing Patient? - When to Use Corticosteroids? - Should we Use Remdesivir? - What about Convalescent Plasma? - How do we Manage Thromboprophylaxis? How do you Identify and Triage Patients at Risk for Severe Disease? In an ideal world, we would be able to assess newly admitted patients with COVID-19 to predict the risk of getting critically ill in the course of the disease. Apart from a proper clinical assessment, JAMA published the COVID-GRAM Risk Score to address this problem. They used a cohort of 1590 patients to develop this score and validated this with a cohort of 710 patients. From 72 potential predictors, ten variables were independent predictive factors and were included in the risk score. The practicability in a clinical setting is not clear yet, and as any predictive score, there are several limitations when it comes to assessing a single patient instead of a cohort. The COVID-GRAM Score Calculator can be accessed via the following link: http://118.126.104.170/ Early identification of COVID-19 patients at risk for severe disease would be helpful for management. Every clinic/ ICU should have a triage and risk assessment tool at hand.
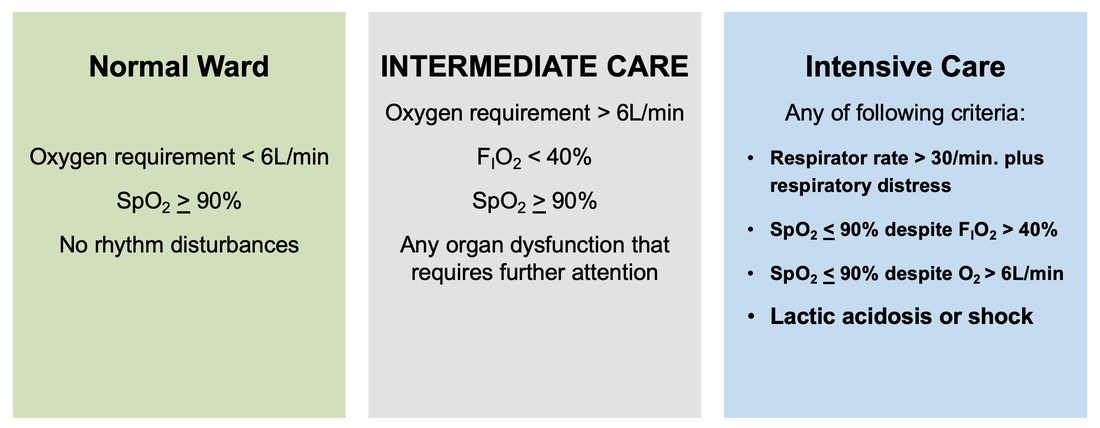
For triage, we use the following simple criteria: As a predictive assessment tool for severe disease the COVID-GRAM Calculator can be used:
What about High Flow Nasal Cannulas (HFNC) and Non-Invasive Ventilation (NIV)? Especially at the beginning during the first wave of the pandemic, the use of HFNC and NIV was often avoided due to aerosolisation fear. Many ICU's tended to intubate their patients with respiratory failure relatively early. The lack of ventilators in some areas and reports that invasive ventilation is associated with high mortality (Zhou F, Lancet 2020; 395:1054) led to a constant change in management. KEEP IN MIND: Randomised-controlled studies for the treatment of COVID-19 patients with HFNC and NIV lack until now! Aerosolisation remains a big concern for health care workers (Niedermann MS; Am J Respir Crit Care Med 2020; 201:1019, Wu Z; JAMA 2020, February 24) and the amount of leakage flows is highly variable (Winck JC; Pulmonology 2020, April 20). Experience during the year 2020 showed, that most critical care providers have moved to use NIV and HFNC more frequently than initially. Proper personal protection equipment is essential and minimises risk for health care providers. Some evidence supports this approach (Avdeev SN, Am J Em Med AJEM Volume 39, p 154-157). NIV and HFNC is feasible in patients with COVID-19 and acute hypoxemic respiratory failure, even outside the ICU Helmet-NIV, leakage-free masks (non-vented masks) and double hose systems with virus-proof filters seem to be advantageous in this respect (Pfeiffer M; Pneumologie 2020, April 22). It is recommended that patients under HFNC should wear a surgical face mask over their cannulas Helmet NIV might advantageous compared to Mask NIV, though evidence is limited. (Patel BK et al. JAMA 2016. PMID: 27179847, single center study, trial stopped early, larger randomized-controlled studies awaited). KEEP IN MIND: Generally, there is only minimal evidence regarding the therapeutic benefit of these measures compared to their risks for the environment due to aerosolisation. Whether HFNC and NIV itself might produce self-inflicted lung injury (SILI) to some extend is not fully understood! Following patients should be considered for intubation and invasive ventilation - Severe hypoxemia (PaO2/FiO2 <150mmHg or respiratory rate >30/min) - Persistent or worsening respiratory failure (i.e. O2 sat <88%, RR > 36/min) - Neurologic deterioration - Intolerance of face mask or helmet - Airway bleeding - Copious respiratory secretions Should we Prone Position the Spontaneously Breathing Patient?Since the publication of Guerin C et al. (N Engl J Med 2013; 368:2159) prone positioning of patients with moderate to severe ARDS has become standard procedure in ICU's around the world. It is, therefore, evident that this treatment modality seems appropriate for COVID-19-induced lung injury, too. Trying to avoid intubations, clinicians rose the question, whether a prone position in the spontaneous breathing patient could avoid the need for invasive ventilation or even improve outcome. Ding L et al. (Crit Care 2020; 24:289) published a small multicenter study including 20 patients, whereas in 11 patients intubation could be avoided by prone positioning patients under NIV or HFNC. Telias et al. published an JAMA editorial (JAMA. 2020;323(22):2265-2267). He states that the prone position can improve oxygenation and can potentially result in less injurious ventilation. Unfortunately, this does not necessarily equate to lung protection and a better outcome. While improved oxygenation might prevent clinicians from intubating a patient, delayed intubation might worsen the patient's outcome. Regarding some evidence showing improved oxygenation during prone position, there are reasons to give it a try (Caputo ND et al. Acad Emerg Med Published online April 22, 2020). In the hypoxemic patient with no relevant respiratory distress awake prone positioning is a valid option - Use nasal cannulas or HFNC first - If comfortable enough, ask the patient to self-prone - Encourage the patient to remain in the prone position as long as well tolerated - Patients need close nursing and appropriate monitoring - Select prone positioning mattresses might be of help When to Use CorticosteroidsPatients with COVID-19 often show a biphasic course of the disease. The first phase is characterised by profound virus replication which decreases significantly after 5-7 days. After 7-10 days, a second phase develops in which an excessive or dysfunctional immune response can appear. This can lead to ARDS and multi-organ failure, which might be tackled by immunomodulating therapy. The largest, pragmatic randomised control trial we have at this stage is RECOVERY, performed in 176 hospitals around the UK and including more than 6400 patients (RECOVERY Collaborative group, N Engl J Med, July 17, 2020). COVID-19 patients that required oxygen or mechanical ventilation and presented with symptoms for at least seven days showed a significant reduction in 28-day mortality when treated with 6 mg Dexamethason OD for up to 10 days. Patients in the early viremic phase or patients that not required any oxygen performed worse with Dexamethasone. A broader insight into this topic brings a meta-analysis from JAMA in September 2020, including seven studies: DEXA-COVID19, CoDEX, RECOVERY, CAPE COVID, COVID STEROID, REMAP-CAP and Steroids-SARI. They ended up looking at 1703 patients and found a significant reduction in 28-day mortality when treated with steroids compared to placebo. Patients with COVID 19 that require oxygen, HFNC, NIV, mechanical ventilation or ECMO should be treated with steroids In patients not requiring oxygen, there is a trend towards harm when giving steroids - In these situations, steroids are NOT indicated Should we Use Remdesivir?Brief: Evidence in regards to the treatment with remdesivir is scattered and inconclusive. In the largest randomised control triad available so far is ACTT-1 looking at about 1600 patients (Beigel JH et al. N Engl J Med 2020; 383:1813-1826). In a few words, remdesivir showed a trend towards a 4-5 day shorter time to recovery, but not if symptoms existed for more than nine days. There was no significant influence on mortality, except maybe for patients requiring oxygen but not any help in ventilation. If at all, remdesivir might provide some advantage in a very selected patient group, but even this remains debatable. For this reason, many consider remdesivir the 'Tamiflu for COVID-19'. Two other papers remain to be mentioned briefly: Wang et al. (The Lancet; April 29) presented results from a relatively small study which was terminated early and showed no statistically significant clinical benefits of remdesivir - except for a trend towards a shorter duration of illness. Goldmann JD et al. presented the so-called '5 versus 10 days study', a phase 3 multicentre study with 397 patients. The primary outcome was their clinical status on day 14, secondary outcome patients with adverse events. Interestingly a 5-day course of remdesivir resulted in a better clinical outcome that a 10-day course. Again, It did not show any benefit compared to placebo. Remdesivir - The "Tamiflu for COVID-19" There is insufficient evidence to recommend the use of Remdesivir strongly. It is expensive, and if used, maybe there is only a short time window reasonable to act. Should We Use ECMO?During the early phase of the pandemic, first reports raised some concern that ECMO in COVID-19 patients might be associated with very high mortality (Henry BM et al. J Crit Care; 58:27). In the meanwhile, though we have new results from a more extensive cohort study looking at data from the Extracorporeal Life Support Organisation (ELSO, Barbaro RP et al. Lancet Volume 396, ISSUE 10257) The investigators looked at 1035 COVID-19 patients from 36 countries that were treated with ECMO (mean age 49 years, 74% male). 70% of all patients had relevant co-morbidities. The median time of ECMO support was 14 days. The incidence of in-hospital mortality 90 days after the initiation of ECMO was 37·4%. Mortality was 39% in patients with a final disposition of death or hospital discharge. These results are comparable with earlier mult-centre studies with patients suffering from non-COVID-19 ARDS (Combes A et al. N Engl J Med 2018; 378:1965). A retrospective cohort study from France looking at 83 patients treated with ECMO showed a probability to die after 60 days of 31%. Mortality at the time of the last follow-up was 36% (Schmidt et al. Lancet Respir Med 2020; 8:1121-1131). Various Societies recommend the use of ECMO in COVID-19 patients with treatment-refractory lung failure (Surviving Sepsis Campaign, ESICM, SCCCM and ELSO, WHO) Regarding the ongoing pandemic and limited resources, uniform indication and selection criteria for ECMO use should be available What about Convalescent Plasma?After a negative small randomised control trial (Li L et al. JAMA. 2020;324(5):460-470), a controversial Emergency Use Authorisation was granted by the FDA on 23.8.20 due to an observational study with a favourable effect on mortality with a high specific IgG content and onset less than days after symptom onset (Joyner MJ et al. MedRxiv; https://doi.org/10.1101/2020.08.12.20169359 - non peer-reviewed). At this stage the use of covalescent plasma can not be recommended How do we Manage Thromboprophylaxis?COVID 19 undoubtedly causes an inflammatory state that seems to trigger thrombotic activation in the venous and the arterial circulation. Thromboembolic complications are common, but the evidence is not robust on whether prophylactic or therapeutic doses should be used. Patients often have a significant elevation of D-dimers, an acute phase reactant representing the severity of disease rather than the dosage of thromboprophylaxis. One observational study looking at 1716 patients found no improved outcomes among in-hospital patients with COVID-19 when treated with therapeutic anticoagulation compared to prophylactic dosing. Moreover, patients who were started on anticoagulation for COVID-19 without evidence of thrombosis, new VTE, or new atrial fibrillation had worse outcomes compared to patients who were on prophylactic anticoagulation (Patel NG et al. Thrombosis Update; Volume 2, 2021, 100027) A case-based review of current literature and the COVID-19 specific coagulopathy end with the same finding that all in-hospital patients should receive prophylactic thromboprophylaxis. Whether a higher dose of prophylactic anticoagulation may be more effective is currently unknown (Chen EC et al. Oncologist. 2020 Oct; 25(10): e1500–e1508.). A small and retrospective study with 152 patients showed a lower risk of death and a lower cumulative incidence of thromboembolic events in patients with respiratory failure when a high-dose thromboprophylaxis was used. (Jonmarker S et al. Critical Care volume 24, Article number: 653 (2020)). Evidence supports the use of prophylactic thromboprophylaxis in patients with COVID-19 Whether a higher dose of anticoagulation might be more effective is currently unknown The W.H.O. has repeatedly warned that antibiotic resistance is one of the biggest threats to global health today. Among all measures we can take to try and reduce this problem, merely avoiding unnecessary treatments is maybe one of the most effective. It is therefore pleasing that another piece of good evidence has been published, supporting the avoidance of antibiotics in the event of non-complicated diverticulitis (defined as non-perforated diverticulitis with a Hinchey 1a grade in computed tomography). The investigators performed a randomized, placebo-controlled, double-blind trial in which they compared 180 patients with non-complicated diverticulitis to receive either cefuroxime, metronidazole, and amoxicillin/clavulanic acid or placebo. They found No significant difference in the median time of hospital stay (primary outcome). Also, there were no significant differences between groups in adverse events, readmission to the hospital within one week, and readmission to the hospital within 30 days. These findings complement other studies indicating that observational treatment without antibiotics can be considered appropriate in patients with uncomplicated diverticulitis. The headlines in the news 2017 were remarkable indeed: "Doctor believes he has found the cure for sepsis..." or "Doctor says improvised 'cure' for sepsis has had remarkable results". Dr. Paul Marik described his observation in an interview in 2017, where he mentions several cases of sepsis that have almost miraculously responded to the application of vitamin c (watch here: Interview on WAVY TV). He even continues, that since then they see "the same thing over and over again". This implicated that these results were reproducible. He finally stated that the current data at that stage were "impressive" and that there was enough basic science to show that it works. Vitamin C has many interesting properties that theoretically could be on benefit in sepsis. (read here: Crit☁ post on Vitamin C). Its application was already proposed for the treatment of other diseases like the common cold of Influenza. Despite some moderate positive influence observed, these results could not be reproduced in trials. While the news picked up on this story as a miracle drug, Paul Marik et al. published their results of a before-and-after single-centre, retrospective cohort study in Chest 2017. In this paper, they compared 47 patients with sepsis that received the metabolic cocktail (Vitamin C 1.5g 6-hourly, hydrocortisone 50mg 6-hourly and thiamine 200mg 12-hourly) to 47 patients which did not - notably in a non-double-blinded, non-randomized fashion. Their results showed overall hospital mortality of 8.5% with the 'cocktail' and 40.4% without its application. This publication was reason enough to launch a small war of faith about sense and nonsense of this cocktail for sepsis. The VITAMINS Trial - First FailureSince 2017 a tiny bunch of studies were published, many of them with significant limitations like a small number of patients, often not randomized-controlled and with conflicting results. Nabil Habib T, Ahmed I (2017) Early Adjuvant Intravenous Vitamin C Treatment in Septic Shock may Resolve the Vasopressor Dependence. Int J Microbiol Adv Immunol. 05(1), 77-81. Shin et al. J Clin Med. 2019 Jan; 8(1): 102. Fowler et al. JAMA. 2019 Oct 1;322(13):1261-1270. Fujii et al. have just now published the first more substantial and rigorous trial taking a closer look at the influence on vitamin c in sepsis. They performed an international, multicenter, randomized-controlled open label trial In which they enrolled 211 patients with septic shock admitted to an ICU. They compared Treatment with Vitamin C 1.5g 6-hourly IV, hydrocortisone 50mg 6-hourly IV and thiamin 200mg 12-hourly IV to Hydrocortisone 50mg 6-hourly IV only. They found No difference in time alive and time free of vasopressors (primary endpoint) and No difference 28 days or 90 days mortality (secondary endpoint) This first study on a larger scale, unfortunately, disappoints. More trials are on the way and might give a clearer picture of this topic to come to a final decision eventually. For the moment it is appropriate to state:
Just as a reminder: Guidelines recommend against the routine use of glucocorticoids in patients with sepsis. However, corticosteroid therapy is appropriate in patients with septic shock that is refractory to adequate fluid resuscitation and vasopressor administration. Fujii et al. JAMA. Published online January 17, 2020. doi:10.1001/jama.2019.22176  Patients who have survived critical illness are at increased risk for long term morbidity and mortality. Maybe we tend to forget this fact, as we lose sight of these patients when they leave our unit. But this is especially true for ICU patients aged 65 and older! There have been clues that influenza vaccination might reduce morbidity after surviving critical illness and Christiansen et al. have looked exactly into this topic. The investigators examined whether an influenza vaccination (flu shot) affects the 1-year risk of myocardial infarction, stroke, heart failure, pneumonia, and death among ICU survivors aged 65 and older. The investigators Performed a nationwide population-based cohort study They used the Danish Intensive Care Database To evaluate a total 89'818 ICU survivors from 2005 until 2015 It is noteworthy that Influenza vaccinated patients (these were 39% of all) were older, had more chronic diseases and used more prescription medications! Their findings show that 1. Influenza vaccinated patients showed an 8% decreased risk of death and a 16% reduced risk of hospitalisation for stroke within one year 2. Cardiac surgery patients were the subgroup that profited most 3. Unfortunately, no significant association was found for the risk of hospitalisation for myocardial infarction, heart failure or pneumonia. The flu shot saves lives! This is another strong hint, that the influenza vaccination is clearly of benefit to all adults aged 65 and older. This is especially true for ICU survivors! Christiansen at al. Intensive Care Med 2019 Jul;45(7):957-967. Also worth mentioning: Not only influenza A but also Influenza B infection can pose a risk for severe secondary infection in previously healthy and younger persons. Aebi et al. BMC Infect Dis 2010 Oct 27;10:308.  The European Society for Clinical Microbiology and Infectious Diseases has now released new guidelines on the diagnosis and treatment of biofilm infections. Written for clinical microbiologists and infectious disease specialists this paper is a MUST READ for anyone involved in treating critically ill patients. These guidelines outline the nature and properties of biofilms and and their implications on mostly chronic infections caused. As biofilms are very common in critically ill patients it is important to know what specific problems you might encounter, how to proceed and perform a proper diagnosis and what are the essential bits and pieces in the prevention and treatment of biofilm infections. The article is OPEN ACCESS: Clin Microbiol Infect. 2015 Jan 14. pii: S1198-743X(14)00090-1.  Almost exactly one year ago the Cochrane Library published an intervention review on the prevention and treatment of influenza with neuraminidase inhibitors in adults and children. The reason for this review was the fact that many countries stockpile these drugs and the WHO classified them as an essential medicine. Jefferson et al. used the data of 46 trials with oseltamivir or zanamivir for this review. They basically conclude that: - Both drugs shorten the duration of symptoms of influenza-like symptoms by less than a day - Oseltamivir did not affect the number of hospitalizations - Prophylaxis trials showed a reduced risk of symptomatic influenza in individuals and households, but no definite conclusion can be made - Oseltamivir use was associated though with nausea, vomiting, headaches, renal and psychiatric events ...and finally write: 'The influenza virus-specific mechanism of action proposed by the producers does not fit the clinical evidence'. This review certainly undermined the importance of oseltamivir for many of us. The Cochrane review though did not look at outcomes like mortality, but the Lancet Respiratory Medicine did! Stella G at al. have now published a large systematic review which included 29'234 patients from 78 studies during the period from 2009 to 2014. Their findings come rather surprisingly: - Compared with no treatment, neuraminidase inhibitor treatment (irrespective of timing) was associated with a reduction in mortality risk - Compared with later treatment, early treatment (within 2 days of symptom onset) was associated with a reduction in mortality risk - The reduction in mortality risk was observed when treatment was started up to 5 days of symptoms onset There still seem to be some good reasons to use oseltamivir in critically ill patients with suspected or proven influenza... up to 5 days of symptoms onset! Jefferson T et al. The Cochrane Collaboration, Published Online: 10 APR 2014 The Cochrane Collaboration News Release 10 April 2014 Muthuri, Stella G et al. The Lancet Respiratory Medicine , Volume 2 , Issue 5 , 395 - 404  Microbiologically confirmed ventilator-associated pneumonia (VAP) or ventilator-associated conditions (VAC, e.g. worsening oxygenation) in intubated patients remains a major concern in ICU's. VAP is defined as a hospital-acquired pneumonia which develops within 48-72 hours after endotracheal intubation. To prevent this complication ICU's uniformly have adapted the VAP-bundle, a bunch of measures aiming to prevent ventilator-associated pneumonia. Unfortunately the evidence of the VAP-bundle is not as robust as one might think it is. Here's the evidence of some elements of the VAP bundle: - Elevation of the head to bed 45° (low evidence) - Daily sedation interruptions (the impact on reducing VAP has not been shown so far) - Daily oral chlorhexidine rinses (low evidence) ... it's most likely the combination of measures that is of benefit to the patient... hopefully! But hold on, there is another intervention that finally brings quite some evidence with it! Active suctioning of the subglottic area, where nasal-oral secretions gather and create a rich culture medium for all sorts of micro-organisms, also aims to reduce the incidence of VAP. In contrast to the classical VAP-bundle the evidence here is strongly in favour for these devices! In 2005 four registrars in cardiothoracic surgery looked into this topic and summarised their efforts online on Best Evidence Topics, best bets.org. In this blog they review 13 relevant articles on the use of subglottic suctioning and conclude: subglottic suction significantly reduces the incidence of VAP in high risk patients - which means a NNT of 8 if ventilated for more than 3 days. They also mention that this measure is cost effective, despite the more expensive tubes. In the same year Dezfulian et al. presented a systematic meta-analysis of randomized trials in the American Journal of Medicine. They ended up with 5 studies including 869 patients. They also came to the conclusion that subglottic secretion drainage is effective in preventing VAP in patients expected to be ventilated for more than 72 hours. In 2011 Hallais et al. looked into the issue of cost-effectiveness with a cost-benefit analysis. Even when assuming the most pessimistic scenario of VAP incidence and costs the replacement of conventional ventilation with continuous subglottic suctioning would still be cost-effective. In 2011 Muscedere et al. published an 'official' review article in Critical Care Medicine and also ended up with 13 randomised clinical trial, most of them the same 'BestBETs' had already identified 6 years before. It is therefore not surprising to see that they also found a highly significant reduction in VAP. They were also able to demonstrate a reduction in ICU length of stay and duration of mechanical ventilation, although the strength of this association was weakened by heterogeneity of study results. We finally would like to mention the latest randomised controlled trial on this topic which was published in Critical Care Medicine this January 2015. Damas et al. randomly assigned 352 patients to either receive subglottic suctioning or not. Again sublottic suctioning significantly reduced VAP prevalence and therefore also antibiotic use. At least we have identified one area in critical care where an impressive pile of evidence supporting the use of subglottic suctioning in long-term intubated patients is present... and even better: cost-effective analyses also come out in great favour for this measure! Take-home message: Subglottic suctioning does prevent VAP in patients likely to be ventilated more than (48-) 72 hours and should be used in these situations. Review BestBETs 2005 Dezfulian C et al. Am J Med. 2005 Jan;118(1):11-8 Hallais C. et al. Infect Control Hosp Epidemiol. 2011 Feb;32(2):131-5 Muscedere J et al. Crit Care Med. 2011 Vol. 39, No. 8 Damas P et al. Crit Care Med. 2015 Jan;43(1):22-30  Just recently in 2014 the WHO has requested to develop a draft global action plan to combat emergent antimicrobial resistance (AMR). AMR is present in all parts of the world, new resistance mechanisms emerge and spread globally. And most importantly: Patients with infections by drug-resistant bacteria are generally at risk of worse clinical outcome and death. On the background of this the recent publication in Nature by Ling et al. is remarkable as it might offer the key to a new antimicrobial weapon in the near future. Teixobactin is the name of a macrocylic peptide representing a new class of antibiotics. It appears to be potent bactericidal agent against a broad panel of bacterial pathogens, especially gram-positive bacteria including MRSA, enterococci and VRE as well as M. tuberculosis, C. difficile and Anthrax. Teixobactin inhibits cell wall synthesis and most remarkably showed no development of resistance so far. Teixobactin is produced by E. terrae, a microorganism discovered in the soil of a grassy field in Maine. As mentioned in the article, these 'uncultured' bacteria make up approximately 99% of all species in external environments, and are an untapped source of new antibiotics. An interesting article, especially if you want to see what's going on outside the hospital! Ling LL et al. Nature 2015; doi:10.1038/nature14098  Ventilator associated pneumonia (VAP) is a problem in ICU around the world and methicillin-resistant Staphylococcus aureus (MRSA) is the most common multi-drug resistant pathogen to deal with. Current guidelines mostly recommend vancomycin as a first line treatment and linezolid as an alternative, considering both drugs at a similar level of efficacy. The question remained whether linezolid might be superior to vancomycin. So far only one prospective, randomised, double-blind trial looked at this question and found a better success rate for linezolid, which was not statistically significant though. To look at this issue the IMPACT-HAP investigators (Improving Medicine through Pathway Assessment of Critical Therapy in Hospital Acquired Pneumonia) performed a multicenter, retrospective, observational study on 188 patients in 5 hospitals of the U.S. They found a significantly higher success rate with linezolid compared to vancomycin in the means of improvement or resolution of the signs and symptoms of VAP (primary endpoint). The study did not identify any difference though between linezolid- and vancomycin-treated patients in regards to mortality, development of thrombocytopenia, anaemia, or nephrotoxicity, days of mechanical ventilation or length of stay ion ICU or the hospital itself (secondary outcomes). Looking into the trial there appear to be several confounding reasons why patients treated with linezolid had better clinical success rate like less severity of sickness in linezolid patients, possible suboptimal vancomycin through levels etc. Overall there seems no good reasons at this stage to change current guidelines. Wunderink RG et al. Linezolid in methicillin-resistant Staphylococcus aureus nosocomial pneumonia, Clin Infect Dis; 2012, 54:621–629 Peyrani P et al. Crit Care 2014; 18:R118 doi:10.1186/cc13914  Antibiotic-associated diarrhoea and antibiotic induced Clostridium difficile diarrhoea are a constant problem in the ICU, especially in the elderly patient. There is still some debate going on about prescribing lactobacilli or bifidobacteria for the prevention and treatment of this sort of complication. In this Lancet multi centre, randomised, double-blind, placebo-controlled, pragmatic, efficacy trial this was studied on almost 3000 patients: 10.8% diarrhoea with lactobacilli or bifidobacteria versus 10.4% diarrhoea in the placebo group. No difference! One drug less to prescribe... Lancet 2013 Oct 12;382(9900):1249-57  This months issue of the American Journal of Respiratory and Critical Care Medicine presents a retrospective cohort study comparing patients with acute exacerbation of COPD receiving either lower-dose methylprednisolone (<240mg/d) or high-dose methylprednisolone (>240mg/d). They looked at 17'239 patients. The primary outcome was mortality. Despite the possibility of some selection bias they conclude that high doses of methylprednisolone are associated with worse outcomes and more frequent adverse effects (like prolonged hospital and ICU length of stay, higher hospital costs, increased length of invasive ventilation, increased need for insulin therapy and higher rate of fungal infections). Mortality itself did not significantly differ. It is remarkable to note that in this study doses below 240mg of methylprednisolone are considered low-dose. This is equivalent to 300mg of prednisolone and is relatively high for exacerbations of COPD. As we mentioned in a post in November 2013 the REDUCE trial in JAMA compared 5 days to 14 days of steroids in exacerbated COPD. The dosage used there was 40mg of prednisone. The results showed that a 5-day treatment was non-inferior to a 14-day treatment with regard to re-exacerbation within 6 months but significantly reduced glucocorticoid exposure. In summary it seems to be advisable to use lower doses and short treatment periods in acute exacerbated COPD. Am J Respir Crit Care Med. 2014 May 1;189(9):1052-64 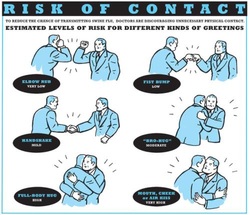 After the 2013 publication by Ghareed et al. (see BIJC post here) on fist bumps in the health care setting in order to prevent transmission of pathogens JAMA now joins in the discussion. Sklansky M et al. published a viewpoint on the banning of the handshake from the health care setting. In their paper they point out that the hands of health care workers often serve as vectors for transmission of organisms and disease. The fact is highlighted that adherence of health care providers with hand hygiene remains rather low and that handshakes have shown to be able to transmit pathogens. In their article they draw parallels between the ban of handshakes in a health care setting and the ban of smoking in public places and finally offer a variety of alternative greetings methods like: the 'hand wave' and placement of the right palm over the heart, or the Namaste gesture also practiced in yoga around the world. This offers an interesting viewpoint worth reading indeed but I might add a few remarks and questions to this article. Apart from the fact that I still struggle to follow the link between hand shakes and smoking in public and would like to highlight following: - The link between pathogen transmission by handshakes and consecutive patient outcome is totally unclear. At this stage there is no evidence indicating that handshakes themselves impose a serious threat to patients. - Banning handshakes in hospitals might sound like a good idea, but the main problem remains unaffected. Multi-resistant bacterias are the logical result of inappropriate prescription and usage of antibiotics. It certainly is advisable to prevent the spread of these pathogens but it would be better to prevent their man made evolution. - Physical contact with patients in the ICU is an essential part in patient care (e.g. nursing or medical examination) and socialising might be even more important when you're unwell. Of course contact isolation has been found to help prevent the retransmission of pathogens. We tend to forget though that all these measures at the same time might have other unintended consequences. From 1999 to 2003 three articles showed that patients in contact isolation got half as many visits from health care providers resulting in 20% less contact time (Morgan DJ et al. Infect Control Hosp Epidemiol. 2013;34(1):69-73). Remarkably, similar effects were found also a decade later. Evidence has continued to accumulate that patients on contact precautions may experience worse outcomes, including more delirium, more depression, worse discharge instructions, and less smoking cessation counselling. Withholding a handshake sounds simple but might actually further contribute to patient's isolation and there is also some research out there actually showing on how important this gesture actually might be (Dolcos S et al. J Cogn Neurosci 2012 Dec;24(12):2292-305). The first sentence of the Conclusion by Sklansky et al. reads as follows: 'Banning the handshake from the health care environment may require further study to confirm and better describe the link between handshake-related transmission of pathogens and disease.'... I couldn't agree more! I think we might have to be very careful on already starting to talk about 'hand shake free zones' as long as there are so many unanswered questions. Many things have been done in the past to prevent infections and finally have been proven to be completely inutile (e.g. changing peripheral lines after three days, read post here). Maybe we she focus more on avoiding overprescribing antibiotics instead. What do you think...? Sklansky et al. JAMA. Published online May 15, 2014. doi:10.1001/jama.2014.4675 The picture displayed above is take from the New York Times  A situation often encountered in hospitals... also in Ireland. You have to urgently review a patient on the ward but are stuck in theatre. Of course you would like to get changed first (as hospital policy asks you to) but there are no scrubs available anymore or there are no white coats to cover your scrubs, or ... so you end up going to the ward in your scrubs... as a walking threat to patients? Apart form the fact that changing your clothes might be appropriate to do there is this recent article of Hee at al. in Anaesthesia giving you a little chance for forgiveness. Although small in numbers these researches found no evidence that visits to ward or office significantly increase bacterial contamination of scrub suits. Hee HI et al. Anaesthesia April 18 2014 Picture above taken from the US series 'Scrubs' One of the Big Mysteries Solved: How to Correctly Prescribe the Duration of Antibiotic Treatments2/5/2014
 Just this week the World Health Organisation WHO has issued a warning that resistance of organisms to antibiotics will become one of the biggest challenges of the upcoming decade. Indeed, the correct prescription of antibiotics is crucial for successful treatment and the WHO states that completing the full length of the treatment is just as important. But what is actually the correct length of treatment for all the different antibiotics and diseases? How many ward rounds on ICU's have I spent with microbiologists (the maybe most important specialists on our sides!) wondering on how they always had a straight answer on the correct length of treatment. 7 days, 10 days or sometimes 21 days... a little mystery to most intensivists, until now! Hitchhiking though the the wide space of the internet I finally found secret to this question. Back in the year 2010 Paul E. Sax, a Professor of Medicine at Harvard Medical School him self, posted an excellent blog for the NEJM Journal Watch website. Inspired by a New York Time article by Harvard Professor Daniel Gilbert he finally gave insight into one of the great mysteries of medicine: To figure out how long antibiotics need to be given, use the following rules:
That did not occur by chance Wow, not much more I can add! Paul E. Sax, NEJM Journal Watch HIV/AIDS Clinical Care, October 22nd 2010 NYT article by Daniel Gilbert from October 2010 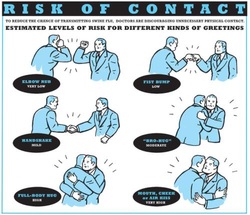 Some studies are fascinating and disconcerting at the same time... but at least they’re good for a smile! In this paper by Ghareeb et al. the authors addressed the question whether a handshake or a fist bump is more effective in preventing or reducing pathogen transmission in the setting of a hospital, where most of us work of course. Unsurprisingly they found that using the fist bump instead of handshakes made skin contact 2.7 times shorter and this reduced spread of bacteria. They concluded that the fist bump is an effective alternative to the handshake in the hospital setting and that bumping might lead to decreased transmission of bacteria and improved health and safety of patients and health care workers alike. So shall we all start bumping around the hospital? We performed an observational study in our medium sized ICU in Galway (without approval of any committee and of course without any statistical evidence to be presented, actually just out of interest and for fun). We might also mention beforehand that working atmosphere in our unit is very good and nurses and doctors work very closely together. Interestingly, neither nursing staff nor doctors shake hands when they meet and greet each other in the ICU. The only occasions handshakes were observed are when relatives are met for a discussion or a representative of some company comes in for a visit. In regards of the study mentioned above I have some serious concerns: I wonder how many health care professionals out there would welcome a family fist bumping that has come in to discuss an end-of-life issue for instance. Also when representing the unit towards someone outside of hospital a fist bump carries some substantial risk of giving a very unprofessional impression... at least in Europe! And in very few occasions also a warm hug will be irreplaceable by some awkward bump. Social behavior is also a way on communicating... and anyhow, who pays for such studies? Ghareeb PA et al. J Hosp Infect. 2013 Dec;85(4):321-3 Picture displayed above is taken from the New York Times |
Search
|





 RSS Feed
RSS Feed


