|
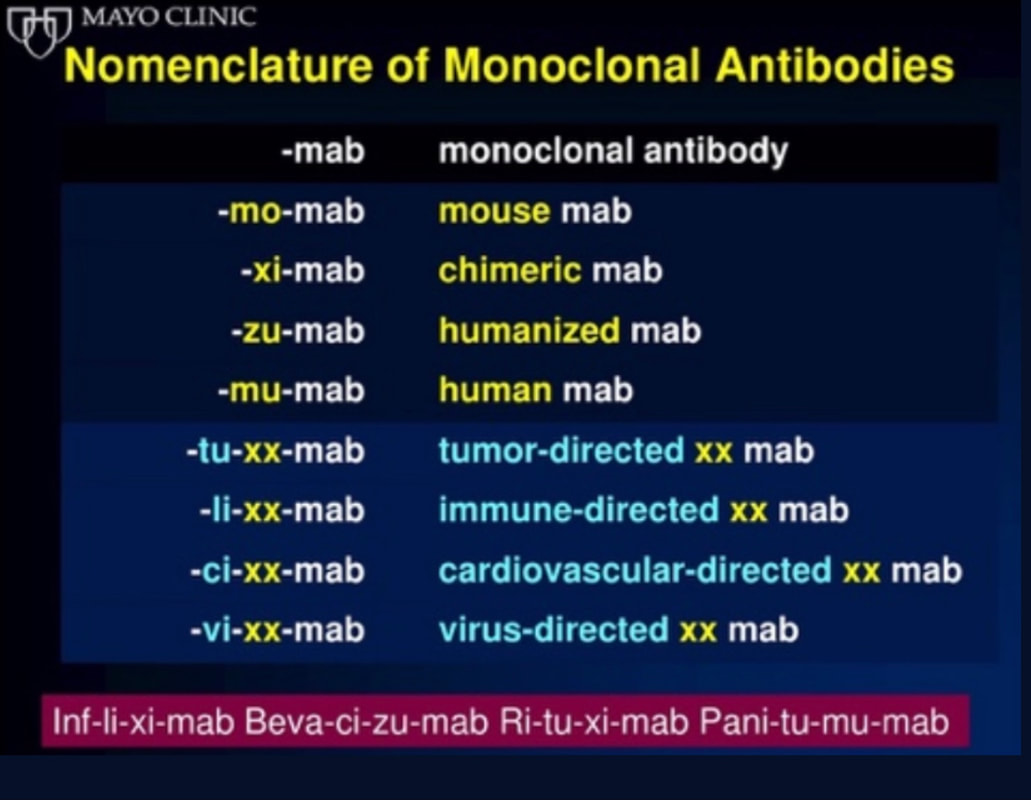
This single slide turn you into an expert in the nomenclature of monoclonal antibodies, but also helps to understand quickly what sort of medication your patient is treated with. Share and Care!
The W.H.O. has repeatedly warned that antibiotic resistance is one of the biggest threats to global health today. Among all measures we can take to try and reduce this problem, merely avoiding unnecessary treatments is maybe one of the most effective. It is therefore pleasing that another piece of good evidence has been published, supporting the avoidance of antibiotics in the event of non-complicated diverticulitis (defined as non-perforated diverticulitis with a Hinchey 1a grade in computed tomography). The investigators performed a randomized, placebo-controlled, double-blind trial in which they compared 180 patients with non-complicated diverticulitis to receive either cefuroxime, metronidazole, and amoxicillin/clavulanic acid or placebo. They found No significant difference in the median time of hospital stay (primary outcome). Also, there were no significant differences between groups in adverse events, readmission to the hospital within one week, and readmission to the hospital within 30 days. These findings complement other studies indicating that observational treatment without antibiotics can be considered appropriate in patients with uncomplicated diverticulitis. The headlines in the news 2017 were remarkable indeed: "Doctor believes he has found the cure for sepsis..." or "Doctor says improvised 'cure' for sepsis has had remarkable results". Dr. Paul Marik described his observation in an interview in 2017, where he mentions several cases of sepsis that have almost miraculously responded to the application of vitamin c (watch here: Interview on WAVY TV). He even continues, that since then they see "the same thing over and over again". This implicated that these results were reproducible. He finally stated that the current data at that stage were "impressive" and that there was enough basic science to show that it works. Vitamin C has many interesting properties that theoretically could be on benefit in sepsis. (read here: Crit☁ post on Vitamin C). Its application was already proposed for the treatment of other diseases like the common cold of Influenza. Despite some moderate positive influence observed, these results could not be reproduced in trials. While the news picked up on this story as a miracle drug, Paul Marik et al. published their results of a before-and-after single-centre, retrospective cohort study in Chest 2017. In this paper, they compared 47 patients with sepsis that received the metabolic cocktail (Vitamin C 1.5g 6-hourly, hydrocortisone 50mg 6-hourly and thiamine 200mg 12-hourly) to 47 patients which did not - notably in a non-double-blinded, non-randomized fashion. Their results showed overall hospital mortality of 8.5% with the 'cocktail' and 40.4% without its application. This publication was reason enough to launch a small war of faith about sense and nonsense of this cocktail for sepsis. The VITAMINS Trial - First FailureSince 2017 a tiny bunch of studies were published, many of them with significant limitations like a small number of patients, often not randomized-controlled and with conflicting results. Nabil Habib T, Ahmed I (2017) Early Adjuvant Intravenous Vitamin C Treatment in Septic Shock may Resolve the Vasopressor Dependence. Int J Microbiol Adv Immunol. 05(1), 77-81. Shin et al. J Clin Med. 2019 Jan; 8(1): 102. Fowler et al. JAMA. 2019 Oct 1;322(13):1261-1270. Fujii et al. have just now published the first more substantial and rigorous trial taking a closer look at the influence on vitamin c in sepsis. They performed an international, multicenter, randomized-controlled open label trial In which they enrolled 211 patients with septic shock admitted to an ICU. They compared Treatment with Vitamin C 1.5g 6-hourly IV, hydrocortisone 50mg 6-hourly IV and thiamin 200mg 12-hourly IV to Hydrocortisone 50mg 6-hourly IV only. They found No difference in time alive and time free of vasopressors (primary endpoint) and No difference 28 days or 90 days mortality (secondary endpoint) This first study on a larger scale, unfortunately, disappoints. More trials are on the way and might give a clearer picture of this topic to come to a final decision eventually. For the moment it is appropriate to state:
Just as a reminder: Guidelines recommend against the routine use of glucocorticoids in patients with sepsis. However, corticosteroid therapy is appropriate in patients with septic shock that is refractory to adequate fluid resuscitation and vasopressor administration. Fujii et al. JAMA. Published online January 17, 2020. doi:10.1001/jama.2019.22176  Die Pocket-Cards 'Toxine und Antidots' stehen nun frei zum Download zur Verfügung. Sie wurden als praktische Hilfe für den klinischen Alltag in Deutsch zusammengestellt und können auch als pdf weiter unten bezogen werden. Feedback und Anregungen jederzeit gerne in den 'comments'! Download die Pocket Cards hier:
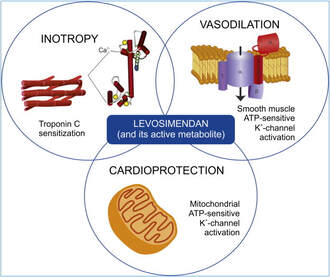
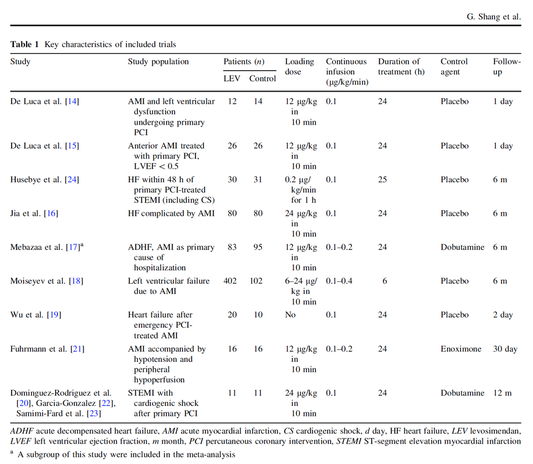
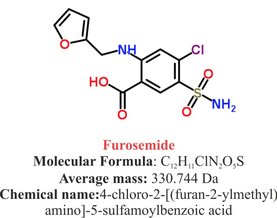
 Acute decompensated heart failure comes in many ways as remains a challenge for optimal treatment since various conditions can cause it, e.g. advanced chronic heart failure, cardiogenic- and septic-shock, cardiac- and non-cardiac surgery, etc. Among many other interventions, the infusion of positive inotropes is often used on one side and vasodilators on the other side to stabilize the situation and improve cardiac function. Despite all these measures, the re-admission rate and mortality remain a significant problem among these patients. While Levosimendan is still under investigation in the U.S., it is used worldwide for the short-term treatment of acutely decompensated severe chronic heart failure, especially when other treatment options have failed. Some recommend the usage of this drug in a different setting like sepsis-related heart failure or coronary heart disease. How does Levosimendan work? Levosimendan has some remarkable properties, which are mainly caused by three mechanisms: 1. Enhancement of the calcium sensitivity of the myofilament by binding to troponin C. 2. Opening of adenosine triphosphate-sensitive potassium (KATP) channels in vasculature smooth muscle. 3. Opening of mitochondrial KATP channels. These mechanisms result in a positive inotropy of the heart, an increase in stroke volume (SV) and therefore, cardiac output (CO). Besides, it's vasodilatory properties seems beneficial for coronary perfusion and reduce pulmonary capillary wedge pressure (PCWP). All these effects do not appear to induce an unfavourable energy balance in the myocardial cell, and also the oxygen demand is not increased. So what's the Evidence? The Small Bits and Pieces First publications back in the 90ies and early 2000s were indicative for the properties of levosimendan. Three studies were randomized and double-blinded but very low in patient numbers and therefore not powered enough to provide substantial evidence. Their statements were also not concerning patient outcome or mortality, but its results confirmed that levosimendan enhances cardiac output without oxygen wasting, is well tolerated and leads to favorable hemodynamic effects. Lilleberg et al. Eur Heart J. 1998;19:660–668. Ukkonen et al. Clin Pharmacol Ther. 2000;68:522–531. Nieminen et al. J Am Coll Cardiol. 2000;36:1903–1912. In 2003 Kivikko et al. published further data from another small trial indication that its beneficial effects (decreases in left and right heart filling pressures and in SVR, as well as increases in stroke volume and cardiac index) are maintained for at least 24 hours after discontinuation of a 24-hour infusion. At this point, it is worth mentioning that the author published these findings as a current employee of Orion Pharma, which manufactures levosimendan. Kivikko et al. Circulation. 2003;107:81–86. Further data indicated that its effect might be sustained for up to at least a week, although also this study included only 22 patients! 8. Lilleberg et al. Eur J Heart Fail. 2007;9:75–82. A Lancet publication in 2002 by Follath et al. presented a multicentre, randomized, double-blind trial in which they compared the effect of levosimendan to dobutamine in patients that were admitted to a hospital with low-output heart failure and were judged to require hemodynamic monitoring and treatment with an intravenous inotropic agent. In these 203 patients, levosimendan had a consistently better effect than dobutamine on the individual hemodynamic variables at the end of the 24 h treatment period (cardiac output and pulmonary capillary wedge pressure). The change in clinical symptoms like fatigue and dyspnoea were not significantly different, though. Interestingly, for the first time, the levosimendan group showed lower mortality than in the dobutamine group for up to 180 days. However, it's important to mention its absence of placebo control and its rather small sample size. Follath et al. Lancet. 2002;360:196–202. The Big Lumps The SURVIVE Study In the SURVIVE study Mebazaa et al. compared the efficacy and safety of intravenous levosimendan or dobutamine in patients hospitalized with acute decompensated heart failure who required inotropic support. They performed a randomized, double-blind trial at 75 centers in 9 countries in which they evaluated 1327 patients hospitalized with acute decompensated heart failure who required inotropic support They found that the addition of levosimendan to standard therapy resulted in fewer deaths compared with dobutamine, especially in the first few weeks after treatment but Levosimendan did not significantly reduce all-cause mortality at 180 days or affect any secondary clinical outcomes The REVIVE Studies - Heart Failure In the Randomized Multicenter Evaluation of Intravenous Levosimendan Efficacy studies (REVIVE I and REVIVE II) the efficacy of levosimendan on symptoms of heart failure during five days after starting a 24h trial drug infusion was assessed. Each patient's clinical course over 5 days was determined by a composite of the patient's self-assessment of symptoms together with a physician's assessment of the occurrence of clinical deterioration. The primary endpoint of the study was a new clinical composite endpoint (improvement, unchanged or worse - including death) derived from studies in chronic heart failure and first evaluated in 100 ADHF patients in the REVIVE I pilot study. In REVIVE II the investigators performed one of the first large, prospective, randomized, double-blind, controlled trials in which they evaluated 600 patients admitted at 103 sites in the United States, Australia, and Israel with worsening heart failure and dyspnea at rest despite treatment with intravenous diuretics, and left ventricular ejection fraction of < 35% measured within the last year whereas patients were randomized to either receive levosimendan for 24h or standard treatment alone They found that after 5 days - more patients receiving levosimendan experienced improvement compared with those who were on placebo (19.4% vs 14.6%, respectively, a 33% relative increase; P = .015) - fewer patients receiving levosimendan worsened compared with patients who were on placebo (19.4% vs 27.2%, respectively), a 29% relative decrease, and - fewer patients receiving levosimendan required rescue therapy (15.1%) vs placebo (26.2%), a 42% relative decrease. Among other secondary endpoints, levosimendan improved B-type natriuretic peptide (BNP) levels, length of hospital stay and dyspnoea at 6 hours But mortality at 90 days did not differ significantly between treatment arms (a secondary endpoint) and the most common treatment-emergent cardiovascular adverse events were more frequent with levosimendan, including hypotension (50% vs 36%), ventricular tachycardia (25% vs 17%), and atrial fibrillation (8% vs 2%). The LeoPARDS-Trial - Sepsis In this study investigators wanted to know whether in adult patients who have sepsis the application of levosimendan reduces the incidence and severity of acute organ dysfunction compared with placebo. They performed a Randomised, double-blind, placebo-controlled multi-centre trial in 34 general ICUs in the UK in which they evaluated 516 patients with suspected or confirmed infection and 2 or more SIRS criteria who were dependent on vasopressors for at least four hours to maintain their blood pressure. They compared the intravenous infusion of levosimendan or placebo for 24 hours in addition to standard care and found: - No significant difference in the daily Sequential Organ Failure Assessment (SOFA) score up to day 28 (primary outcome) - No statistical difference on death at 28 days, at ICU discharge and hospital discharge (secondary outcome) - And: The use of levosimendan was associated with more hemodynamic instability, lower mean arterial pressures, therefore more need for noradrenalin at 24h and significantly more supraventricular tachyarrhythmias (all secondary outcomes). The CHEETAH Trial - Cardiac Surgery The question in CHEETAH was if levosimendan compared to placebo reduces mortality in patients undergoing cardiac surgery with left ventricular dysfunktion. This time Landoni et al. performed a Multi-centre, randomised, placebo-controlledand parallel group designed trial in which they evaluated 506 patients scheduled for cardiac surgery with peri-operative cardiovascular dysfunction defined as pre-operative left ventricular ejection fraction (LVEF) < 25%, pre-operative intra-aortic balloon pump (IABP), intra- or post-operative (within 24 hours) IABP or significant inotropic requirement They compared Levosimendan infusion continued for up to 48 hours (or until ICU discharge) or placebo and found No difference in mortality at thirty 30 days (primary outcome) And no difference in survival over time, renal replacement therapy, the median duration of mechanical ventilation, the median hospital stay and interruptions due to adverse events (all secondary outcomes). Any Other Clues? In contrast to these clinically somewhat discouraging results Shang et al. have published a meta-analysis in 2017 of randomized controlled trials looking at the usage of levosimendan in patients with heart failure, cardiogenic shock and acute coronary syndrome. They ended up looking at a total of nine studies, most of them low in patient numbers and comparing levosimendan to either placebo or other drugs (dobutamine and enoximone). According to these data the authors conclude that levosimendan is associated with reduced total mortality, decreased incidence of worsening HF, and improved hemodynamic outcomes and does not increase the risk of adverse events except for hypotension in patients with HF (including CS) complicating ACS. Thus, levosimendan should be recommended for routine clinical application in these patients. Am J Cardiovasc Drugs 2017 Dec;17(6):453-463. According to current evidence - Levosimendan has interesting mechanisms of action and successfully seems to enhance cardiac output without additional oxygen wasting. This includes decreased right and left ventricular filling pressures, decreased SVR, as well as increases in stroke volume and cardiac index. Also, PCWP is decreased. - In terms of various patient groups, patients with acute heart failure or acute on chronic heart failure appear to benefit in terms of clinical symptoms, if at all. - Unfortunately, there is no convincing clinical evidence that levosimendan has any benefit in long term outcomes in terms of mortality. - And, levosimendan seems to be associated with some potential treatment-emergent cardiovascular adverse events like hypotension, supraventricular and ventricular arrhythmia.  Just recently a 75-year-old gentleman was admitted to our unit as he remained unresponsive after short procedural sedation with Propofol. Although he was hemodynamically stable and showed proper spontaneous breathing at any time, the intervention team was concerned about his condition. On arrival to our ICU, the patient was noted to be still unresponsive. He showed no reaction to painful stimulation. The Pupils were rather wide, but symmetrical in size and showed a prompt response to light. Brain stem reflexes were present and peripheral reflexes were triggerable. His breathing pattern was regular, and saturation levels were above 96% on room air. A blood gas analysis showed a normal pH and normal CO2-levels. As usual, the common reflexes started to kick in, and some suggested to go for a CT-scan of his head to out-rule some significant complications. Luckily enough, close observation revealed some slow improvement of his alertness, and we considered the possible diagnosis of a central anticholinergic syndrome (CAS). After the application of 1mg of physostigmine (2x0.5mg) intravenously the patient almost promptly awoke and had an uneventful stay on our unit. This case just reminded me of these many patients in anaesthesia that inexplicably show delayed awakening after sedation or a general anaesthetic. In fact, it is estimated that the incidence of central anticholinergic syndrome is around 8- 12 % following general anaesthetic and lesser with regional anaesthesia. What is a Central Anticholinergic Syndrome? Classically the central anticholinergic syndrome (CAS) describes a condition where a substance causes a competitive antagonism of acetylcholine (ACh) at peripheral and central muscarinic receptors. Initially, these were plants containing atropine, hyoscyamine and scopolamine. There are four muscarinic receptors: - M1 mainly in the central nervous system (responsible for delirium when antagonised) - M2 in the brain and heart - M3 in the salivary glands and - M4 in the brain and lungs The PERIPHERAL SYNDROME presents with: - Dry mouth - Difficulty swallowing (lack of saliva) - Photophobia and blurred vision (due to dilated pupils) - Dry skin, fever - Reduced bowel sounds and urinary retention The CENTRAL SYNDROME presents with: - Agitation, agitated delirium, visual and auditory hallucinations - Hypoactive delirium may also occur, this seems to be more common though in the postoperative setting The clinical diagnosis of a CAS is more straightforward when typical peripheral symptoms accompany central signs. The Problem with the "Silent" Postoperative CASThe clinical diagnosis of a CAS is often straight forward when typical central symptoms accompany peripheral symptoms. The problems are patients in the postoperative setting, in which the patient often presents with somewhat atypical central symptoms and often minimal or even no peripheral symptoms at all. Especially in this setting, the anticholinergic syndrome may be accompanied by sedation or coma. The mechanisms causative for this phenomenon are not well understood but might include greater tolerance at peripheral receptors, longer persistence at central receptors or greater CNS susceptibility due to age or disease. Postoperative CAS is often associated with atypical central symptoms and minimal or even no peripheral signs at all! Sedation and coma are often observed in this setting. What Drugs Cause CAS?Common anticholinergics agents should be more accurately referred to as antimuscarinics, as these agents do not generally block nicotinic receptors. The Problem is that many currently used drugs in anaesthesia and critical care are also known to cause this syndrome. This includes: Benzodiazepines, opioids, phenothiazines, butyrophenones, ketamine, etomidate, propofol, nitrous oxide, and volatile inhaled anaesthetics! How Can I Diagnose a "Silent" Postoperative CAS?The fact that postoperative CAS often lacks the presence of peripheral symptoms makes the diagnosis challenging. The different presentation of the syndrome ranging from somnolence, confusion, amnesia, delayed recovery, stupor, coma to agitation, hallucinations, dysarthria, ataxia, delirium makes it difficult to diagnose accurately. It is, therefore, a diagnosis of exclusion! The most helpful tool you have is physostigmine! The prompt arousal of a patient after the application of intravenous physostigmine is highly suggestive of a postoperative central anticholinergic syndrome. How to Use PhysostigmineWhen dealing with prolonged somnolence or unexplained agitation following any form of anaesthesia, make sure to check and monitor vital signs and provide basic or advanced life support if necessary. Exclude common causes first (e.g. overhang of sedatives or opioids, persistent muscular paralysis, hypoxemia, hypercapnia, hypoglycemia and other). If common causes can be excluded and CAS is a probable diagnosis, you should consider the application of intravenous physostigmine. Physostigmine in recommended doses is considered safe! Possible adverse effects are considered unlikely. Studies found cholinergic symptoms sometimes to be mild, and these adverse effects are more an indication of probable excessive doses rather than an established safety concern. For postoperative CAS a dose of 1mg of physostigmine i/v is recommended. This dose can be divided into two doses of 0.5mg i/v if required. The maximum dose recommended in this setting is 2mg i/v.  Beware: You are giving Physostigmine Salicylat - Do not give to patients with aspirin allergy! 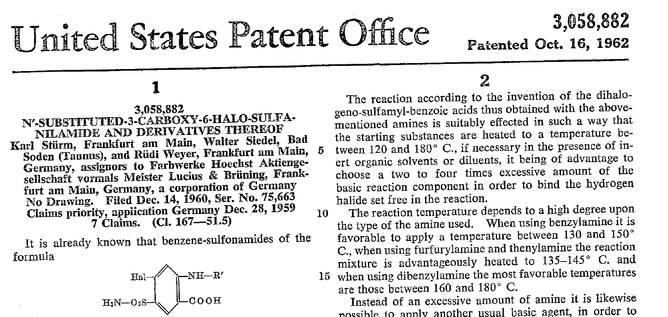 Just recently our ICU team was called to the wards to look at a 74-year-old gentleman with sudden shortness of breath and low peripheral saturation. He was known to suffer from hypertensive heart disease and now presented with acute pulmonary oedema. After giving oxygen over a non-rebreathing mask, he was administered furosemide (Lasix) intravenously and brought to the unit for non-invasive ventilation. Interestingly a discussion started on whether giving Lasix as a first line agent in the acute setting of pulmonary oedema is beneficial or not. A quick look into to current literature gave no clear answer and reading further into the topic revealed unusual properties of Lasix we hadn't been really aware of so far. We all use and love Lasix, but do we really know the drug? The Beginning of Lasix Furosemide (sometimes also called frusemide) was first developed by 'Farbwerke Hoechst AG' in Frankfurt am Main, Germany, a company that was founded back in the year 1863. Karl Stürm, Walter Siedel and Rüdi Weyer set the basis with the invention of N-substituted-3-Carboxy-6-Halo-Sulfanilamide, and it's derivates, one of them being furosemide. The researchers soon noticed its saluretic (sodium Na, potassium K and chloride Cl) and diuretic effect in almost equivalent proportions. As these substances did not cause any acidosis nor alkalosis, they suggested their future use for the treatment of oedema and hypertension.  The Naming of Furosemide Researchers soon noticed that the diuretic effect of furosemide lasted for about 6 hours... 'LAsts for SIX hours'... and therefore gave it the name: LASIX! What is Furosemide Furosemide is an organic anion from the group of loop diuretics (as are bumetanide and torasemide) and is sold under the brand name of Lasix©. Its indications are for the treatment of oedema due to heart or liver disease as well as kidney disease. It is also used for the treatment of mild or moderate hypertension. Furosemide has become one of the cornerstones in the treatment of heart failure. How does it work? Furosemide can be applied by oral intake as a tablet or as an intravenous injection. Once in the bloodstream, it is predominantly bound to proteins (>90%). Loop diuretics do not undergo glomerular filtration. In fact, they pass the glomerulus and are actively secreted across proximal tubular cells by organic anion transporters and the multidrug resistance-associated protein 4 (area A). It is important to know that non-steroidal anti-inflammatory drugs (NSAID) and endogenous uremic anions compete with this loop diuretic secretion and can cause 'diuretic resistance'. Once loop diuretics have reached the tubular system, they bind to sodium-potassium-chloride co-transporters (NKCC2) in the ascending limb of the loop of Henle and block the reabsorption of these ions directly (area B). Further down at the macula densa they inhibit the same co-transporter (area B) thereby stimulating renin secretion and inhibiting tubuloglomerular feedback. This results in preserved glomerular filtration despite increased salt delivery to the macula densa. All this finally results in the loss of sodium, chloride and potassium and therefore loss of water. Other Effects Furosemide also interacts with other sodium-potassium-chloride co-transporters (NKCC1) elsewhere in the body: - Blocking NKCC1 in the ear probably explains the ototoxicity of loop diuretics - Blocking NKCC1 in smooth muscle cells causes vasodilation - Blocking NKCC1 in the afferent arteriole and near the macula densa elevates renin secretion and the generation of angiotensin II These complex interactions on haemodynamics explain that the net response in each patient might be different. On the one hand, loop diuretics dilate blood vessels directly and increase the level of vasodilatory prostaglandins. On the other hand, some of these effects counteract each other making it difficult to predict which effect will finally predominate. Many studies have looked closer into the vasoactive properties of furosemide. Current evidence indicates that it has a systemic venodilator effect which actually reduced preload acutely. The same investigators found a reduction in the right atrial pressure and the pulmonary capillary wedge pressure, presumably reflecting the systemic venodilator effect of furosemide. While the acute venodilator effect may be beneficial to the failing heart, its action on arteries might be detrimental. Several studies have shown that in patients with chronic heart failure furosemide causes arterial vasoconstriction. Also, there is one study showing that pulmonary vascular resistance in healthy volunteers rose significantly. Francis GS et al. described how the administration of furosemide actually led to decreased LV function, increased LV filling pressures, increases in MAP, SVR, plasma renin activity, and plasma noradrenaline levels. Beneficial venodilator response predominates over arterial vasoconstriction in patients with (1) myocardial infarction and (2) salt depleted volunteers. Venous relaxant effect has not been demonstrated in patients with chronic heart failure. In this setting detrimental arterial vasoconstriction seems to predominate. Pardeep S et al. Br J Clin Pharmacol. 2000 Jul; 50(1): 9–13. Francis GS et al. Ann Int Med 1985; 103(1): 1-6. Pharmacological Properties Administered furosemide orally has a limited and highly variable bioavailability. The diuretic effect starts within the first hour, and the duration of action is around 6 hours (4-8 hours). Injected furosemide intravenously is approximately twice as potent on a per-milligramme basis as oral doses. In acute decompensated heart failure sodium retention becomes more avid and higher peak levels might be required to become more effective. This can be achieved by giving furosemide intravenously. Once a loop diuretic is administered, the excretion of sodium chloride is increased for several hours. This is then followed by a period of very low sodium excretion resulting in a so-called 'post-diuretic retention'. How to use Furosemide for Acute Decompensated Heart Failure (ADHF) So far for the basics of furosemide, but what about its usage for acutely decompensated heart failure? Should furosemide be given as soon as possible or not? The 2013 ACCF/AHA guidelines for the management of patients with heart failure give diuretics a class I recommendation. The evidence behind these recommendations though is level B or level C only! So these recommendations are not really helpful to answer this question. The authors in UpToDate® mention diuretics directly after the use of oxygen. For patients with evidence of volume overload their recommendation is to give loop diuretics immediately (Grade 1B) as there is evidence that in this setting this may improve outcomes. They also suggest that patients with ADHF usually are volume overloaded, therefore indicating that most patients should receive diuretics ASAP. The only exception they mention where some delay in inducing diuresis might be required is in patients with severe hypotension or cardiogenic shock. There is reasonable doubt that patients with ADHF are usually volume overloaded, as suggested by UpToDate®. Zile MR et al. demonstrated that while most patients with acute pulmonary oedema have increased filling pressures, most did not have significant increases from their dry weight on presentation! Fallick et al. actually argue that it isn't fluid gain but rather shift in fluids from other compartments, mainly shift from the splanchnic circulation, which usually is very compliant. And as mentioned above, there is evidence that giving a straight shot of furosemide might actually influence haemodynamics negatively in different ways (decreased LV function, increased LV filling pressures, increases in MAP, SVR, plasma renin activity and plasma noradrenaline levels). In conclusion there is no straight forward answer to this question, but I would put it down as follows: - Furosemide should not be routinely used for the immediate treatment of acute decompensated heart failure (ADHF)/ acute pulmonary oedema - However, in patients with evidence of volume overload the administration of early furosemide (preferentially given as an intravenous bolus) seems beneficial and improves outcome. But beware, most patients are not volume overloaded! - In urgent situations the focus should be on early non-invasive ventilation and the administration of nitroglycerin! David H et al. N Engl J Med 2017;377:1964-75. Wilson S et al., UpToDate.com 2018 WRITING COMMITTEE MEMBERS, Yancy CW, Jessup M, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation 2013; 128:e240. Zile MR, Bennett TD, St John Sutton M, et al. Circulation 2008 Sep 30;118(14):1433-41 Fallick C et al. Circ Heart Fail 2011; 4: 669-75.
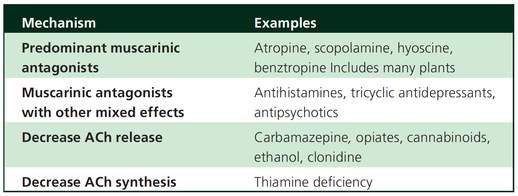
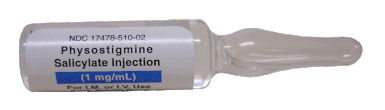
 Postoperative pain and delirium is a common concern and currently approached by different interventions. There is some evidence suggesting that ketamine given intra-operatively might have an influence on postoperative pain and delirium. Some anaesthetists commonly give a single dose of ketamine intra-operatively for exactly this reason. Thumbs up for Ket Ketamine has kept its fascination in various settings, from retrieval medicine onto the the care of critically ill patients in the ICU. Ketamine reduces postoperative markers of inflammation, is a rapid-acting antidepressant drug with an effect lasting for several days and might have neuroprotective properties. Ketamine also has become increasingly popular as an adjunct to other sedatives in the ICU. There is evidence showing that ketamine used in the ICU has the potential to reduce cumulative opioid consumption after surgery (Asad E. et al. J Intensive Care Med December 8 2015 ). Even better: It does not cause any kidney injuries, preserves laryngeal protective reflexes, lower airway resistance and much more... And: Ketamine is cheap and has been used safely for over 50 years by anaesthetists! The Dark Side of Ket But there's the other side of ketamine making all of this a little more complicated. After all, Ketamine is a psychoactive drug and has well known hallucinogenic properties. Developed in the 1960s as a dissociative anaesthetic agent it started to appear on the street in the early 1970s and made its way to the 1980s as Special K, Acid and Super C (Dotson JW et al. J of Drug Abuse, Vol 25, Issue 4, 1995). From a medical point of view there are some worries that these psychotomimetic effects, which are of concern in the critically ill patient, might predispose to delirium (Erstad BL, J Crit Care, Oct 2016, Vol 35, p 145-149). The PODCAST Trial On the background of all this facts this trial revealed some interesting findings. Avidan et al. performed a multicentre, international randomised trial in which they randomly assigned 672 patients undergoing major cardiac and non-cardiac surgery under general anaethesia into three groups to either receive a bolus of placebo (normal saline), low-dose ketamine (0·5 mg/kg), or high dose ketamine (1·0 mg/kg) after induction of anaesthesia, before surgical incision. Participants, clinicians, and investigators were blinded to group assignment. They found NO difference in in the incidence of postoperative delirium among these groups but significantly more postoperative hallucinations and nightmares with increasing ketamine doses compared to placebo This trial seems well performed with an acceptable sample size. The application of a single dose of ketamine before surgery neither prevented delirium nor induced it. With this sample size it seems safe to say that even if ketamine does prevent delirium, its effect would be rather small.
Furthermore, postoperative pain was not influenced by giving a single dose of ketamine and this is in contrast to previous findings and current guidelines. Importantly, most of the previous studies are smaller than this trial, making these findings remarkable. But what really drew my attention was the fact that the appearance of hallucinations and night-mares was increased for at least 3 days after surgery. So if ketamine has no influence on postoperative delirium or pain but does induce hallucinations and nightmares, even 3 days after surgery, current guidelines might have to be revised. The Bottom Line - The application of a subanaesthetic dose of ketamine during surgery to tackle postoperative pain and delirium does not seem to be as effective as previously assumed - The usage of ketamine in this setting even seems to have undesirable side-effects like hallucinations and nightmare - and this effect might even last for up to 3 days! - This trial provides good reasons to look for other options to prevent postoperative delirium! (Like dexmedetomidine? The answer to this question has just been answered: READ HERE!) Sometimes there's this moment you read about medical research in the news... sometimes you read lots of rubbish on medical issues in the news... but sometimes you stop and read, and you don't know what to think. This happened to quite some of us a couple of days ago when reading the headlines in the British Independent: Well, it's not very often you read the term sepsis in the news but the word 'cure' causes estonishment or rather misbelief. Further reading certainly catches your attention: 'A doctor in the US state of Virginia claims to have found his own cure for sepsis' and 'Since then, he has used it to treat 150 sepsis patients. Just one has died of the condition, claims Dr Marik'. And it's not an article from some remote pseude magazine... no, it has been published in 'Chest'! And all this is not due to some novel molecule... it's all about Vitamin C! Thanks to #FOAMed quite some smart brains have looked into this topic already... So here's the most important facts you need to know - in short: What's the Story?Paul Marik et al. have published a single-centre retrospective cohort study in which they have treated 47 consecutive septic patients over a periode of 7 months with intravenous vitamin C (1.5g 6-hourly), hydrocortisone (50mg 6-hourly) and thiamine (200mg 12-hourly) and then compared these patients to 47 septic patients treated in their unit during the preceding 7 months They performed Propensity score matching and found An overall hospital mortality of 40.4% in the control group compared to 8.5% in the intervention group This means An absolute risk reduction of 31.9% and also according to the authors none of the patients in the intervention arm died of sepsis! What Does This Mean?These results are quite amazing on the first look, but there's more behind these numbers. Paul Marik has first of all published an observational study: unblinded, uncontrolled, retrospective and low in patient numbers. There are several limitations that go hand in hand with studies as such and unblinded before-and-after studies have a lot. A major challenge in conducting observational studies is to draw inferences that are acceptably free from influences by overt biases, as well as to assess the influence of potential hidden biases. One of the biggest drawbacks in this current study is the timely/ seasonal difference when patients have been selected. If you are interested to have a closer look on this you should read Dan's blog entry on stemlynsblog.org HERE. Studies like this one are an important part of science, but observational studies are observational... not proof! Why Vitamin C in Sepsis? There is a scientific rationale behind all of this. As mentioned by Paul in his paper vitamin C levels do fall low in sepsis and the most efficient way to administer it is intravenously. The same is true for thiamin which also goes low in up to one third of all septic patients. There are two rather small randomised control trials suggesting that vitamin C is safe in septic patients and might actually be of some degree of benefit for the patient. Vitamin C - Neutralizes free radicals and has therefore antioxydative properties - Is an important conenzyme for the procollagen-proline dioxygenase, which itself is necessary for the biosynthesis of stable collagen in our body. Vitamin C deficiency leeds to unstable collagen and therefore scurvy - Is an important cofactor in the synthesis of steroids like cortisol and catecholamines like dopamine and noradrenalin as well - and it has many more functions that go beyond the scope of this blog entry! However, the importance of vitamin C in the treatment and prevention of diseases like e.g. the common cold or influenza remains highly contrversial. The observation of some moderate positive influence on the course of disease in some studies could not be reproduced in other trials. Under normal circumstances vitamin C deficiency is practically non-existent in Europe, but becomes a fact during sepsis. If this is clinically relevant in septic patients seems plausible but remains to be elucidated. Shailja Chambial, Shailendra Dwivedi, Kamla Kant Shukla, Placheril J. John, and Praveen Sharma. Vitamin C in Disease Prevention and Cure: An Overview. Indian Journal of Clinical Biochemistry. Oktober 2013; 28(4): S. 314–328 H. Hemilä, E. Chalker: Vitamin C for preventing and treating the common cold. Cochrane Database of Systematic Reviews. 2013 R. M. Douglas, E. B. Chalker, B. Treacy: Vitamin C for preventing and treating the common cold. In: Cochrane Database of Systematic Reviews. 2000; 2:CD000980. Another great read into the details: Josh Farkas from pulmcrit More Ifs and ButsSepsis is not a disease, its a clinical syndrome that has physiologic, biologic and biochemical abnormalities caused by a dysregulated inflammatory response to infection. The fact that different definitions have evolved since the early 1990s shows that we still struggle to definde sepsis as a single entity. This is one reason why a single therapy might not always be the best for each diesease causing sepsis. Paul Marik’s publication is interesting and deserves respect. It’s an observational study but provides no evidence by far. Vitamin C might be an interesting novel approach to sepsis but the term ‘cure’ used in the media is inappropriate and misleading. The term ‘cure for sepsis’ also implicates that vitamin C is a cure for all infections causing sepsis and is therefore problematic. The Current Bottom Line- The study published by Marik et al. is purely observational and provides no proof at all. - Just because vitamine C might be safe in Sepsis does not mean this has to be given. At this stage no recommendation can be made for the use of vitamin C in sepsis. - Studies like these are an part of research itself - However, the use of the term 'cure' seem problematic and inappropriate in this context. Marik et. al, J Chest 2017  When filling out the form for a CT scan in you hospital you will not only have to provide clinical information about the patient but almost certainly also the latest creatinine levels. This information is required as many clinicians are worried that IV contrast media might cause iatrogenic acute kidney injury and therefore increased rates of dialysis, renal failure, and death. Despite several reports of contrast-induced nephropathies in the past, the causal relationship between IV contrast media and the development of acute kidney injury has been challenged recently (Read our previous summary HERE).
The major problem is that performing a randomized controlled trial to elucidate the true incidence of contrast-induced nephropathy is considered unethical because of the presumption that contrast media administration is a direct cause of acute kidney injury. While the discussion goes on Hinson et al. have come up with another nice piece of evidence that in emergency situations there is no reason to withhold the application of IV contrast for CT scans when required. In this single-center retrospective cohort study researchers have included a total of 17'934 patient visits to their emergency department over a period of 5 years. They analysed three patient groups that where demographically similar: contrast-enhanced CT, unenhanced CT and no CT scan performed. Patients were included when their initial serum creatinine level was between 35 umol/L and 352 umol/L. Of all CT scans, 57.2 percent were contrast-enhanced. The probability of developing acute kidney injury was 6.8 percent for patients undergoing contrast-enhanced CT, 8.9 percent for patients receiving unenhanced CT and 8.1 percent for patients not receiving CT at all. This proofs to be the largest controlled study of its kind in the emergency department and shows that: In current clinical context, contrast media administration for CT scans is NOT associated with an increased incidence of acute kidney injury. And even though a large randomised controlled trial is still missing it seems safe... To Conclude: There is no reason to withhold the use of IV contrast media in cases where contrast-enhanced CT is indicated to avoid delayed or missed diagnosis of critical disease. Hinson J et al. Annals of Emergency Medicine, 2017; DOI: 10.1016/j.annemergmed.2016.11.021 OPEN ACCESS Crit Cloud Review from 18/01/2015  For the resuscitation out-of-hospital one of the mainstays besides compression and defibrillation ist the application of adrenalin and amiodarone. According to the new ACLS guidelines 2015 these are the only drugs remaining in the treatment for shockable rhythms. While adrenaline is given for maximum vasoconstriction in order to promote coronary perfusion pressure CPP, amiodarone and sometimes lidocaine are used to promote successful defibrillation of shock-refractory ventricular fibrillation VF or pulseless ventricular tachycardia VT. While the usage of these drugs is undoubtedly very effective in patients with existing circulation the effectiveness during resuscitation remains a matter of debate. The Effect of Adrenaline As a matter of fact it has never been proven that adrenalin actually improves long-term outcome. In 2014 Steve Lin and colleagues published a systemativ review on the efficacy of adrenaline in adult out-of-hospital cardiac arrest (OHCA). They were able to show that according to current evidence standard dose adrenaline (1mg) improved rates of survival to hospital admission and return of spontaneous circulation (ROSC) but had no benefit in means of survival to discharge or neurologic outcomes. What about Amiodarone and Lidocaine? Kudenchuck et al. now made the effort to look into the efficacy of amiodarone and lidocaine in the setting of OHCA. Used according to the ACLS guidelines 2016 amidarone is given after the third shock applied when treating a shockable rhythm. Two rather small controlled trials have shown so far that using amidarone actually does increase the likelihood of ROSC and the chance to arrive at a hospital alive. It's impact on survival to hospital discharge and neurologic outcome though remains uncertain. In this randomized, double-blind trial, the investigators compared parenteral amiodarone, lidocaine and saline placebo in adult, non-traumatic, OHCA. They ended up with 3026 patients meeting inclusion criteria and which were randomly assigned to receive amiodarone, lidocaine or saline placebo for treatment. They finally found that neither amiodarone nor lidocaine improved rate of survival to discharge or neurologic outcome significantly. There were also no differences in these outcomes between amiodarone and lidocaine. Across these trial groups also in-hospital care like frequency of coronary catheterisation, therapeutic hypothermia and withdrawal of life-sustaining treatments did not really differ, making a bias due to treatments after admission unlikely. Take Home- This study was not able to show any benefit of amiodarone or lidocaine in the the setting of OHCA in terms of survival to hospital discharge and neurologic outcome
- Amiodarone seems to improve the likelihood of ROSC and survival to hospital admission (similar to adrenaline) - As there are no other options, I believe amiodarone should remain part of the standard treatment for shockable rhythms in OHCA - Lidocaine can be safely removed from CPR sets as there is no benefit of over amiodarone Read here: N Engl J Med 2016;374:1711-22 Resuscitation, June 2014, Vol 85, Issue 6, p 732-740 New ACLS Guidelines 2015, The Changes  As posted on BIJC before, Asad et al. had performed a systematic review on the usage of ketamine as a continuous infusion (>24h) in intensive care patients. The same authors have now published a narrative review providing a more depth discussion about the pharmacological and pharmacokinetic properties of ketamine. Also they present recommendations for dosing and monitoring in an ICU setting. The Goodies of KetCurrent evidence shows that Ketamine... - Has no adverse effects on the gastrointestinal tract (bleeding) and does not cause acute kidney injury (compared to nonsteroidal anti-inflammatory drungs, NSAID's) - Does not negatively influence bowel motility (in contrast to opioids) - Preserves laryngeal protective reflexes - Lowers airway resistance - Increases lung compliance - Is less likely to cause respiratory depression - Is sympathomimetic, facilitates adrenergic transmission and inhibits synaptic catecholamine reuptake, therefore increasing heart rate and blood pressure The Concerns of KetKetamine... - Might increase pulmonary airway pressure and therefore aggravate pulmonary hypertension - Might cause well known psychotomimetic effects which are of concern in the critically ill patient as this might predispose to delirium - Interacts with benzodiazepines via the P450 pathway which could result in drug accumulation and prolonged recovery Concerns Proven Wrong- Ketamine need not to be avoided in patients at risk for seizures, particularly when used for analgosedation for short periods in the ICU setting - Current evidence shows no increased intracranial pressure or associated adverse neurologic outcomes associated with ketamine administration in critically ill patients Take HomeThe use of ketamine for analgosedation in the ICU continues to lack high-level evidence.However, it is effectively used around the globe and remains an attractive alternative agent for appropriately selected patients. Taking current knowledge and evidence into account this is especially true for patients with severe pain unresponsive to conventional therapies.
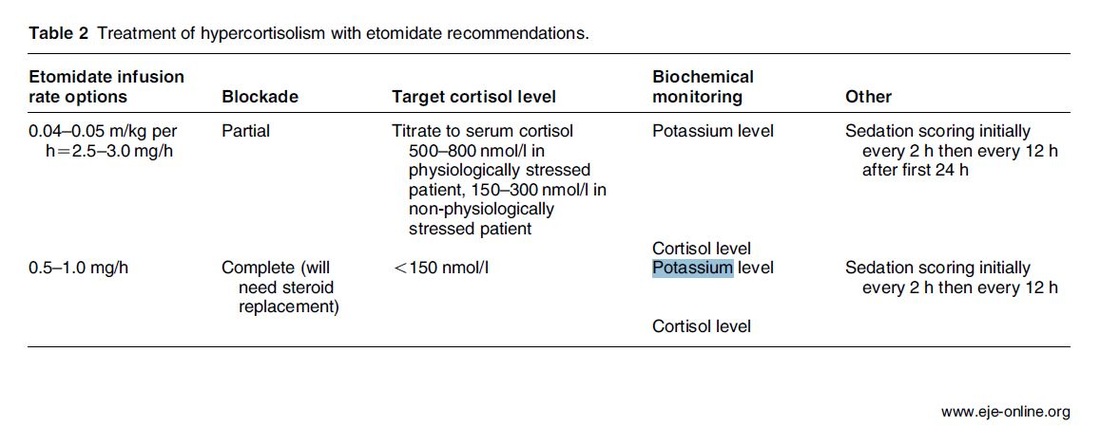
Taking precautions and contraindications into account ketamine is considerably safe and even avoids potentially adverse side effects of other agents used. Erstad BL, J Crit Care, Oct 2016, Vol 35, p 145-149 The Problem An endogenous Cushing's syndrome, mostly caused by an adenoma of the pituitary gland, is associated with significant morbidity and mortality when left untreated. The condition is closely associated to life-threatening infections, diabetes mellitus, hypertension and increased risk associated with surgery. For Cushing's disease the first line therapy is surgical removal of the pituitary tumor. Sometimes though urgent medical therapy is needed first. It has been shown, that surgical risk may be significantly reduced if cortisol concentrations are normalised preoperatively. Conditions requiring urgent cortisol-lowering measures are severe biochemical disturbances (e.g. hypokalaemia), immunosuppression or mental instability. Medical Treatment OptionsKetokonazole (yes, the antifungal agent) and metyrapone are used to suppress adrenal steroidogenesis at enzymatic sites. Both agents carry the risk of postential side effects. Mifepristone, a glucocorticoid receptor antagonist, and pasireotide, a new targeted pituitary therapy, are alternative agents. However, they also have their limits and side effects. EtomidateNow that's where etomidate joins the game. Interestingly, etomidate and ketokonazole are chemically closely related... they are both members of the imidazole family. Etomidate is used as an anaesthetic agent since 1972 and became popular for hemodynamic stability and the lack if histamine release. In 1983 a Lancet article noted an increased mortality when etomidate was used in critically unwell patients. In 1984 an article in Anaesthesia first showed a link to low serum cortisol levels caused by etomidate. Until now the discussion continues, whether a single induction dose actually negatively influences patient outcome. A meta-analysis in 2010 was unable confirm this apprehension and the debate continues. Fact isEtomidate suppresses the production of cortisol by inhibiting the mitochondrial cytochrome p450-dependent adrenal enzyme 11-beta-hydroxylase and therefore lower serum cortisol levels within 12 hours. In higher doses it also blocks side chain cleavage enzymes and also aldosterone synthase. It might even have anti-proliferative effects on adrenal cortical cells. On this basis the idea arose, that etomidate might be a useful therapy for severe hypercortisolaemia. Continuous Etomidate - What's the EvidenceA review article by Preda et al. in 2012 identified 18 publications about the primary therapeutic usage of etomidate in Cushing's syndrome, most of which were case reports. Review of current literature reveals that etomidate indeed suppresses hypercortisolaemia safely and efficiently in patients requiring parenteral therapy. Moreover, etomidate shows a dose-dependent suppression and allows adjustment of the medication to target cortisol levels. At recommended dosages etomidate is considered safe with almost no serious side effects. The authors conclude, that etomidate is a useful therapeutic option in a hospital setting when oral therapy is not tolerated or inappropriate. Take home- Continuous etomidate (in non-hypnotic doses) reduces cortisol concentrations in a dose-dependent manner in both hyper- and eucortisolaemic subjects
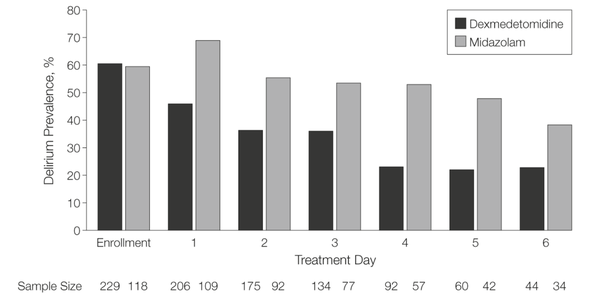
- The application of continuous etomidate in Cushing's disease is safe and efficient - After termination of infusion adrenocortical suppression persists for about 3 hours - The suspicion, that a single dose of etomidate for rapid sequence inductions might negatively influence patient outcome in the critically ill remains a matter of debate J Clin Endocrinol Metab. 1990 May;70(5):1426-30. Preda et al. European Journal of Endocrinology (2012) 167 137-143 OPEN ACCESS Soh et al. Letter to the Editor, European Journal of Endocrinology (2012) 167 727–728 Ge et al. Critical Care201317:R20 OPEN ACCESS  Dexmedetomidine has shaken up the usual sedatives in ICU but remains a matter of debate among intensivists. One question is whether the higher costs compared to midazolam are justified by clinical advantages. There is research available suggesting that dexmedetomidine might be an attractive alternative to standard sedatives especially in regards of time to extubation and costs (Turinen et al., Jacob et al.). This seems to hold true for moderate to light sedation of intubated patients. I've stepped over this prospective, double-blind, randomised trial by Riker et al. in which 68 centres in 5 countries recruited intubated 366 patients to received moderate to light sedation with either dexmedetomidine or midazolam. All patients received daily arousal assessment. Their primary end point was the percentage of time within the target sedation range (RASS score −2 to +1) and this did not differ between the two groups. Looking at the secondary endpoints though make things a lot more interesting. Just before the beginning of the sedation period both groups had a similar prevalence of delirium. During study drug administration though, the effect of dexmedetomidine treatment on delirium was significant. A reduction of 24.9% with dexmedetomidine is rather impressive (see figure below). This effect was even greater in patients who were CAM-ICU-positive at baseline. Finally patients on dexmedetomidine had shorter time to extubation (1.9 days in average) while their length of stay on ICU did not differ. From a safety point of view the most common adverse effect of dexmedetomidine was bradycardia. It's noteworthy that patients on midazolam had more episodes of hypotension and tachycardia. THE BOTTOM LINE - This is another study indicating that dexmedetomidine seems to be beneficial in regards of delirium in mechanically ventilated patients and might speed up time to extubation - Dexmedetomidine is safe in patients where moderate to light sedation is the aim Riker et al. JAMA. 2009;301(5):489-499. doi:10.1001/jama.2009.56 OPEN ACCESS Read more HERE on BIJC  Ketamine's success seems unstoppable: +++ Anaesthesiologists are opening private clinics for off-label infusions of ketamine for depression http://bit.ly/1IGYTcI +++ Dr. Jim Roberts says #ketamine is an ideal treatment for excited #delirium: http://emn.online/Dec15InFocus +++ Major #ketamine treatment trial to start in 2016 http://m.huffpost.com/au/entry/8501942 +++ More impressed every day with low dose ketamine for pain management! https://www.youtube.com/watch?v=DgckjVVBb48 ... Intravenous ketamine is also used in critical care units and to my knowledge most clinicians use ketamine as an adjunct to other sedatives. This might be for patients on mechanical ventilation, intubation procedures or simply as an additive to a patient-controlled analgesia pump. I personally think ketamine is one of the essentials in ICU's, but what does the evidence say. Asad et al. have performed a systematic review on the usage of ketamine as a continuous infusion (>24h) in intensive care patients. The aim was to find evidence in favour for the utilisation of ketamine in the ICU. As a result of this review - current evidence suggests that: - In critically ill postoperative patients ketamine has the potential to reduce the cumulative morphine consumption at 48h compared to morphine only - Several trials show the potential safety of ketamine in regards of cerebral haemodynamics in patients with traumatic brain injury, improved gastrointestinal motility and decreased vasopressor requirements - One observational study and case reports suggest that ketamine is safe, effective and may have a role in patients who are refractory to other therapies Our conclusion: THUMBS UP for ketamine in the ICU Asad E. et al. J Intensive Care Med December 8, 2015 |
Search
|
||||||






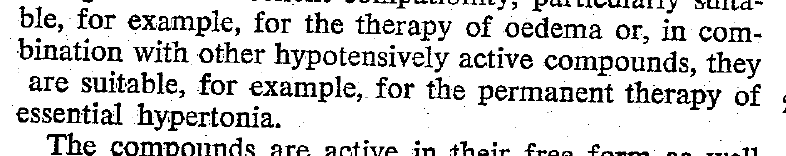



 RSS Feed
RSS Feed


