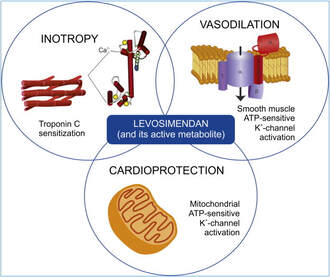
 Acute decompensated heart failure comes in many ways as remains a challenge for optimal treatment since various conditions can cause it, e.g. advanced chronic heart failure, cardiogenic- and septic-shock, cardiac- and non-cardiac surgery, etc. Among many other interventions, the infusion of positive inotropes is often used on one side and vasodilators on the other side to stabilize the situation and improve cardiac function. Despite all these measures, the re-admission rate and mortality remain a significant problem among these patients. While Levosimendan is still under investigation in the U.S., it is used worldwide for the short-term treatment of acutely decompensated severe chronic heart failure, especially when other treatment options have failed. Some recommend the usage of this drug in a different setting like sepsis-related heart failure or coronary heart disease. How does Levosimendan work? Levosimendan has some remarkable properties, which are mainly caused by three mechanisms: 1. Enhancement of the calcium sensitivity of the myofilament by binding to troponin C. 2. Opening of adenosine triphosphate-sensitive potassium (KATP) channels in vasculature smooth muscle. 3. Opening of mitochondrial KATP channels. These mechanisms result in a positive inotropy of the heart, an increase in stroke volume (SV) and therefore, cardiac output (CO). Besides, it's vasodilatory properties seems beneficial for coronary perfusion and reduce pulmonary capillary wedge pressure (PCWP). All these effects do not appear to induce an unfavourable energy balance in the myocardial cell, and also the oxygen demand is not increased. So what's the Evidence? The Small Bits and Pieces First publications back in the 90ies and early 2000s were indicative for the properties of levosimendan. Three studies were randomized and double-blinded but very low in patient numbers and therefore not powered enough to provide substantial evidence. Their statements were also not concerning patient outcome or mortality, but its results confirmed that levosimendan enhances cardiac output without oxygen wasting, is well tolerated and leads to favorable hemodynamic effects. Lilleberg et al. Eur Heart J. 1998;19:660–668. Ukkonen et al. Clin Pharmacol Ther. 2000;68:522–531. Nieminen et al. J Am Coll Cardiol. 2000;36:1903–1912. In 2003 Kivikko et al. published further data from another small trial indication that its beneficial effects (decreases in left and right heart filling pressures and in SVR, as well as increases in stroke volume and cardiac index) are maintained for at least 24 hours after discontinuation of a 24-hour infusion. At this point, it is worth mentioning that the author published these findings as a current employee of Orion Pharma, which manufactures levosimendan. Kivikko et al. Circulation. 2003;107:81–86. Further data indicated that its effect might be sustained for up to at least a week, although also this study included only 22 patients! 8. Lilleberg et al. Eur J Heart Fail. 2007;9:75–82. A Lancet publication in 2002 by Follath et al. presented a multicentre, randomized, double-blind trial in which they compared the effect of levosimendan to dobutamine in patients that were admitted to a hospital with low-output heart failure and were judged to require hemodynamic monitoring and treatment with an intravenous inotropic agent. In these 203 patients, levosimendan had a consistently better effect than dobutamine on the individual hemodynamic variables at the end of the 24 h treatment period (cardiac output and pulmonary capillary wedge pressure). The change in clinical symptoms like fatigue and dyspnoea were not significantly different, though. Interestingly, for the first time, the levosimendan group showed lower mortality than in the dobutamine group for up to 180 days. However, it's important to mention its absence of placebo control and its rather small sample size. Follath et al. Lancet. 2002;360:196–202. The Big Lumps The SURVIVE Study In the SURVIVE study Mebazaa et al. compared the efficacy and safety of intravenous levosimendan or dobutamine in patients hospitalized with acute decompensated heart failure who required inotropic support. They performed a randomized, double-blind trial at 75 centers in 9 countries in which they evaluated 1327 patients hospitalized with acute decompensated heart failure who required inotropic support They found that the addition of levosimendan to standard therapy resulted in fewer deaths compared with dobutamine, especially in the first few weeks after treatment but Levosimendan did not significantly reduce all-cause mortality at 180 days or affect any secondary clinical outcomes The REVIVE Studies - Heart Failure In the Randomized Multicenter Evaluation of Intravenous Levosimendan Efficacy studies (REVIVE I and REVIVE II) the efficacy of levosimendan on symptoms of heart failure during five days after starting a 24h trial drug infusion was assessed. Each patient's clinical course over 5 days was determined by a composite of the patient's self-assessment of symptoms together with a physician's assessment of the occurrence of clinical deterioration. The primary endpoint of the study was a new clinical composite endpoint (improvement, unchanged or worse - including death) derived from studies in chronic heart failure and first evaluated in 100 ADHF patients in the REVIVE I pilot study. In REVIVE II the investigators performed one of the first large, prospective, randomized, double-blind, controlled trials in which they evaluated 600 patients admitted at 103 sites in the United States, Australia, and Israel with worsening heart failure and dyspnea at rest despite treatment with intravenous diuretics, and left ventricular ejection fraction of < 35% measured within the last year whereas patients were randomized to either receive levosimendan for 24h or standard treatment alone They found that after 5 days - more patients receiving levosimendan experienced improvement compared with those who were on placebo (19.4% vs 14.6%, respectively, a 33% relative increase; P = .015) - fewer patients receiving levosimendan worsened compared with patients who were on placebo (19.4% vs 27.2%, respectively), a 29% relative decrease, and - fewer patients receiving levosimendan required rescue therapy (15.1%) vs placebo (26.2%), a 42% relative decrease. Among other secondary endpoints, levosimendan improved B-type natriuretic peptide (BNP) levels, length of hospital stay and dyspnoea at 6 hours But mortality at 90 days did not differ significantly between treatment arms (a secondary endpoint) and the most common treatment-emergent cardiovascular adverse events were more frequent with levosimendan, including hypotension (50% vs 36%), ventricular tachycardia (25% vs 17%), and atrial fibrillation (8% vs 2%). The LeoPARDS-Trial - Sepsis In this study investigators wanted to know whether in adult patients who have sepsis the application of levosimendan reduces the incidence and severity of acute organ dysfunction compared with placebo. They performed a Randomised, double-blind, placebo-controlled multi-centre trial in 34 general ICUs in the UK in which they evaluated 516 patients with suspected or confirmed infection and 2 or more SIRS criteria who were dependent on vasopressors for at least four hours to maintain their blood pressure. They compared the intravenous infusion of levosimendan or placebo for 24 hours in addition to standard care and found: - No significant difference in the daily Sequential Organ Failure Assessment (SOFA) score up to day 28 (primary outcome) - No statistical difference on death at 28 days, at ICU discharge and hospital discharge (secondary outcome) - And: The use of levosimendan was associated with more hemodynamic instability, lower mean arterial pressures, therefore more need for noradrenalin at 24h and significantly more supraventricular tachyarrhythmias (all secondary outcomes). The CHEETAH Trial - Cardiac Surgery The question in CHEETAH was if levosimendan compared to placebo reduces mortality in patients undergoing cardiac surgery with left ventricular dysfunktion. This time Landoni et al. performed a Multi-centre, randomised, placebo-controlledand parallel group designed trial in which they evaluated 506 patients scheduled for cardiac surgery with peri-operative cardiovascular dysfunction defined as pre-operative left ventricular ejection fraction (LVEF) < 25%, pre-operative intra-aortic balloon pump (IABP), intra- or post-operative (within 24 hours) IABP or significant inotropic requirement They compared Levosimendan infusion continued for up to 48 hours (or until ICU discharge) or placebo and found No difference in mortality at thirty 30 days (primary outcome) And no difference in survival over time, renal replacement therapy, the median duration of mechanical ventilation, the median hospital stay and interruptions due to adverse events (all secondary outcomes). Any Other Clues? In contrast to these clinically somewhat discouraging results Shang et al. have published a meta-analysis in 2017 of randomized controlled trials looking at the usage of levosimendan in patients with heart failure, cardiogenic shock and acute coronary syndrome. They ended up looking at a total of nine studies, most of them low in patient numbers and comparing levosimendan to either placebo or other drugs (dobutamine and enoximone). According to these data the authors conclude that levosimendan is associated with reduced total mortality, decreased incidence of worsening HF, and improved hemodynamic outcomes and does not increase the risk of adverse events except for hypotension in patients with HF (including CS) complicating ACS. Thus, levosimendan should be recommended for routine clinical application in these patients. Am J Cardiovasc Drugs 2017 Dec;17(6):453-463. According to current evidence - Levosimendan has interesting mechanisms of action and successfully seems to enhance cardiac output without additional oxygen wasting. This includes decreased right and left ventricular filling pressures, decreased SVR, as well as increases in stroke volume and cardiac index. Also, PCWP is decreased. - In terms of various patient groups, patients with acute heart failure or acute on chronic heart failure appear to benefit in terms of clinical symptoms, if at all. - Unfortunately, there is no convincing clinical evidence that levosimendan has any benefit in long term outcomes in terms of mortality. - And, levosimendan seems to be associated with some potential treatment-emergent cardiovascular adverse events like hypotension, supraventricular and ventricular arrhythmia. Comments are closed.
|
Search
|


 RSS Feed
RSS Feed


