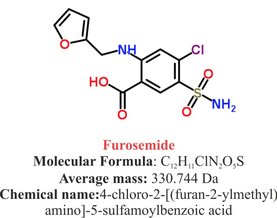
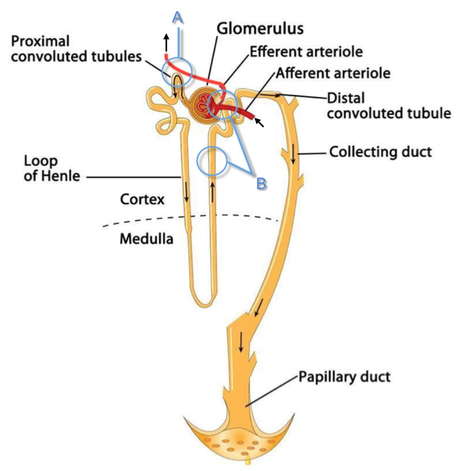
 Just recently our ICU team was called to the wards to look at a 74-year-old gentleman with sudden shortness of breath and low peripheral saturation. He was known to suffer from hypertensive heart disease and now presented with acute pulmonary oedema. After giving oxygen over a non-rebreathing mask, he was administered furosemide (Lasix) intravenously and brought to the unit for non-invasive ventilation. Interestingly a discussion started on whether giving Lasix as a first line agent in the acute setting of pulmonary oedema is beneficial or not. A quick look into to current literature gave no clear answer and reading further into the topic revealed unusual properties of Lasix we hadn't been really aware of so far. We all use and love Lasix, but do we really know the drug? The Beginning of Lasix Furosemide (sometimes also called frusemide) was first developed by 'Farbwerke Hoechst AG' in Frankfurt am Main, Germany, a company that was founded back in the year 1863. Karl Stürm, Walter Siedel and Rüdi Weyer set the basis with the invention of N-substituted-3-Carboxy-6-Halo-Sulfanilamide, and it's derivates, one of them being furosemide. The researchers soon noticed its saluretic (sodium Na, potassium K and chloride Cl) and diuretic effect in almost equivalent proportions. As these substances did not cause any acidosis nor alkalosis, they suggested their future use for the treatment of oedema and hypertension.  The Naming of Furosemide Researchers soon noticed that the diuretic effect of furosemide lasted for about 6 hours... 'LAsts for SIX hours'... and therefore gave it the name: LASIX! What is Furosemide Furosemide is an organic anion from the group of loop diuretics (as are bumetanide and torasemide) and is sold under the brand name of Lasix©. Its indications are for the treatment of oedema due to heart or liver disease as well as kidney disease. It is also used for the treatment of mild or moderate hypertension. Furosemide has become one of the cornerstones in the treatment of heart failure. How does it work? Furosemide can be applied by oral intake as a tablet or as an intravenous injection. Once in the bloodstream, it is predominantly bound to proteins (>90%). Loop diuretics do not undergo glomerular filtration. In fact, they pass the glomerulus and are actively secreted across proximal tubular cells by organic anion transporters and the multidrug resistance-associated protein 4 (area A). It is important to know that non-steroidal anti-inflammatory drugs (NSAID) and endogenous uremic anions compete with this loop diuretic secretion and can cause 'diuretic resistance'. Once loop diuretics have reached the tubular system, they bind to sodium-potassium-chloride co-transporters (NKCC2) in the ascending limb of the loop of Henle and block the reabsorption of these ions directly (area B). Further down at the macula densa they inhibit the same co-transporter (area B) thereby stimulating renin secretion and inhibiting tubuloglomerular feedback. This results in preserved glomerular filtration despite increased salt delivery to the macula densa. All this finally results in the loss of sodium, chloride and potassium and therefore loss of water. Other Effects Furosemide also interacts with other sodium-potassium-chloride co-transporters (NKCC1) elsewhere in the body: - Blocking NKCC1 in the ear probably explains the ototoxicity of loop diuretics - Blocking NKCC1 in smooth muscle cells causes vasodilation - Blocking NKCC1 in the afferent arteriole and near the macula densa elevates renin secretion and the generation of angiotensin II These complex interactions on haemodynamics explain that the net response in each patient might be different. On the one hand, loop diuretics dilate blood vessels directly and increase the level of vasodilatory prostaglandins. On the other hand, some of these effects counteract each other making it difficult to predict which effect will finally predominate. Many studies have looked closer into the vasoactive properties of furosemide. Current evidence indicates that it has a systemic venodilator effect which actually reduced preload acutely. The same investigators found a reduction in the right atrial pressure and the pulmonary capillary wedge pressure, presumably reflecting the systemic venodilator effect of furosemide. While the acute venodilator effect may be beneficial to the failing heart, its action on arteries might be detrimental. Several studies have shown that in patients with chronic heart failure furosemide causes arterial vasoconstriction. Also, there is one study showing that pulmonary vascular resistance in healthy volunteers rose significantly. Francis GS et al. described how the administration of furosemide actually led to decreased LV function, increased LV filling pressures, increases in MAP, SVR, plasma renin activity, and plasma noradrenaline levels. Beneficial venodilator response predominates over arterial vasoconstriction in patients with (1) myocardial infarction and (2) salt depleted volunteers. Venous relaxant effect has not been demonstrated in patients with chronic heart failure. In this setting detrimental arterial vasoconstriction seems to predominate. Pardeep S et al. Br J Clin Pharmacol. 2000 Jul; 50(1): 9–13. Francis GS et al. Ann Int Med 1985; 103(1): 1-6. Pharmacological Properties Administered furosemide orally has a limited and highly variable bioavailability. The diuretic effect starts within the first hour, and the duration of action is around 6 hours (4-8 hours). Injected furosemide intravenously is approximately twice as potent on a per-milligramme basis as oral doses. In acute decompensated heart failure sodium retention becomes more avid and higher peak levels might be required to become more effective. This can be achieved by giving furosemide intravenously. Once a loop diuretic is administered, the excretion of sodium chloride is increased for several hours. This is then followed by a period of very low sodium excretion resulting in a so-called 'post-diuretic retention'. How to use Furosemide for Acute Decompensated Heart Failure (ADHF) So far for the basics of furosemide, but what about its usage for acutely decompensated heart failure? Should furosemide be given as soon as possible or not? The 2013 ACCF/AHA guidelines for the management of patients with heart failure give diuretics a class I recommendation. The evidence behind these recommendations though is level B or level C only! So these recommendations are not really helpful to answer this question. The authors in UpToDate® mention diuretics directly after the use of oxygen. For patients with evidence of volume overload their recommendation is to give loop diuretics immediately (Grade 1B) as there is evidence that in this setting this may improve outcomes. They also suggest that patients with ADHF usually are volume overloaded, therefore indicating that most patients should receive diuretics ASAP. The only exception they mention where some delay in inducing diuresis might be required is in patients with severe hypotension or cardiogenic shock. There is reasonable doubt that patients with ADHF are usually volume overloaded, as suggested by UpToDate®. Zile MR et al. demonstrated that while most patients with acute pulmonary oedema have increased filling pressures, most did not have significant increases from their dry weight on presentation! Fallick et al. actually argue that it isn't fluid gain but rather shift in fluids from other compartments, mainly shift from the splanchnic circulation, which usually is very compliant. And as mentioned above, there is evidence that giving a straight shot of furosemide might actually influence haemodynamics negatively in different ways (decreased LV function, increased LV filling pressures, increases in MAP, SVR, plasma renin activity and plasma noradrenaline levels). In conclusion there is no straight forward answer to this question, but I would put it down as follows: - Furosemide should not be routinely used for the immediate treatment of acute decompensated heart failure (ADHF)/ acute pulmonary oedema - However, in patients with evidence of volume overload the administration of early furosemide (preferentially given as an intravenous bolus) seems beneficial and improves outcome. But beware, most patients are not volume overloaded! - In urgent situations the focus should be on early non-invasive ventilation and the administration of nitroglycerin! David H et al. N Engl J Med 2017;377:1964-75. Wilson S et al., UpToDate.com 2018 WRITING COMMITTEE MEMBERS, Yancy CW, Jessup M, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation 2013; 128:e240. Zile MR, Bennett TD, St John Sutton M, et al. Circulation 2008 Sep 30;118(14):1433-41 Fallick C et al. Circ Heart Fail 2011; 4: 669-75.
 When filling out the form for a CT scan in you hospital you will not only have to provide clinical information about the patient but almost certainly also the latest creatinine levels. This information is required as many clinicians are worried that IV contrast media might cause iatrogenic acute kidney injury and therefore increased rates of dialysis, renal failure, and death. Despite several reports of contrast-induced nephropathies in the past, the causal relationship between IV contrast media and the development of acute kidney injury has been challenged recently (Read our previous summary HERE).
The major problem is that performing a randomized controlled trial to elucidate the true incidence of contrast-induced nephropathy is considered unethical because of the presumption that contrast media administration is a direct cause of acute kidney injury. While the discussion goes on Hinson et al. have come up with another nice piece of evidence that in emergency situations there is no reason to withhold the application of IV contrast for CT scans when required. In this single-center retrospective cohort study researchers have included a total of 17'934 patient visits to their emergency department over a period of 5 years. They analysed three patient groups that where demographically similar: contrast-enhanced CT, unenhanced CT and no CT scan performed. Patients were included when their initial serum creatinine level was between 35 umol/L and 352 umol/L. Of all CT scans, 57.2 percent were contrast-enhanced. The probability of developing acute kidney injury was 6.8 percent for patients undergoing contrast-enhanced CT, 8.9 percent for patients receiving unenhanced CT and 8.1 percent for patients not receiving CT at all. This proofs to be the largest controlled study of its kind in the emergency department and shows that: In current clinical context, contrast media administration for CT scans is NOT associated with an increased incidence of acute kidney injury. And even though a large randomised controlled trial is still missing it seems safe... To Conclude: There is no reason to withhold the use of IV contrast media in cases where contrast-enhanced CT is indicated to avoid delayed or missed diagnosis of critical disease. Hinson J et al. Annals of Emergency Medicine, 2017; DOI: 10.1016/j.annemergmed.2016.11.021 OPEN ACCESS Crit Cloud Review from 18/01/2015  When performing a kidney transplantation nowadays up to 50% of recipients developed a delayed graft function which is defined as the need of dialysis within seven days. The authors of this recently published NEJM-article asked themselves whether mild hypothermia might influence outcome in this regard. In order to answer this question the investigators assigned organ donors after declaration of death according to neurologic criteria into two groups. They were either treated with mild hypothermia (34 to 35°C) or with normothermia (36.5 to 37.5°C). The target temperature was maintained until the patients were transferred to theatre for transplantation. Primary outcome of this trial was delayed graft function among recipients. Secondary outcomes included the rates of individual organs transplanted into each treatment group at the total number of organs transplanted from each donor. This trial had to be stopped early as an interim analysis showed significant efficacy of mild hypothermia. Up to this point a total of 572 patients received a kidney transplant (285 in the hypothermia group and 287 in the normothermia group). 28% of recipients in the hypothemia group developed delayed graft function compared to 39% in the normothermia group. The authors therefore conclude that mild hypothermia significantly reduces the rate of delayed graft functions among recipients.
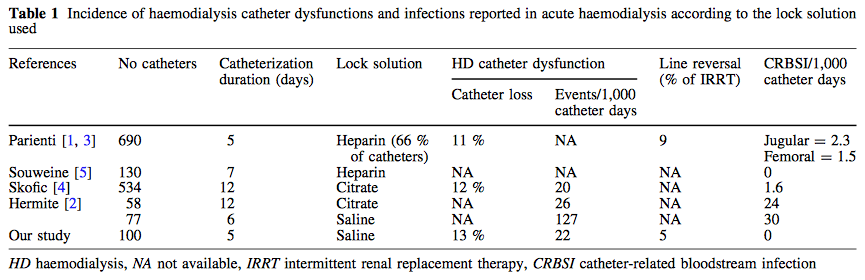
Anyhow, it seems reasonable not to get rid of your cooling devices!  Read more about the controversies of hypothermia in the ICU: The Targeted Temperature Management Trial: Nielsen N, et al. New Engl J Med. 2013 Dec;369(23):2197-206 The 2 trials that introduced therapeutic hypothermia into ICU practice: The Hypothermia After Cardiac Arrest Study Group, Holzer at al. New Engl J Med. 2002 Feb;346(8):549-556 Bernard S.A. et al. New Engl J Med. 2002 Feb;346(8):557-563 Review article on therapeutic hypothermia for non-VF/VT cardiac arrest: Sandroni S. et al. Crit Care Med; 2013;17:215 Pyrexia and neurological outcome: Leary M. et al. Resuscitation. 2013 Aug;84(8):1056-61 BIJC post on: The Effect of Pre-Hospital Cooling: Rather Worrying Results  In a letter to the editor of Intensive Care Medicine Soubirou et al. present the result of a study looking at the efficacy and safety of saline lock solution in maintaining short term hemodialysis catheters patency in ICU. This prospective cohort study looked at 100 double lumen hemodialysis catheters inserted in 75 patients managed with intermitted hemodialysis. At the end of each session the lumens were flushed with normal saline only. The investigators found no difference to 5 other studies using heparin or citrate. Conclusion: Heparin is not necessary in this setting, citrate is an alternative, but saline seems just as good. Soubirou JF et al. Intensive Care Med. 2014 June Iodinated Radiocontrast Agents can cause Kidney Injury... But not as much as we thought they would!12/3/2014
 One major concern when bringing a critically ill patient for a CT scan is the potential for acute kidney injury (AKI) by applying iodinated contrast media intravenously. Post-contrast AKI carries the risk of more permanent renal failure, dialysis and even death. The authors of this review article nicely summarize current evidence on this issue and show, that the risk of AKI secondary to contrast material (particularly when administered intravenously for contrast-enhanced CT) has been exaggerated in the past by older, noncontrolled studies. In fact, by reviewing more recent evidence they come to the conclusion that the risk is almost nonexistent in patients with normal renal function. Even in patients with pre-existing renal insufficiency the risk of secondary contrast-induced AKI is probably much smaller than traditionally assumed. Again they emphasize on the fact that volume expansion is the only preventive strategy with a convincing evidence base. Nevertheless, the benefits of a contrast-enhanced exam still will have to be balanced with the remaining risk of AKI. BioMed Research International. 2014, Article ID 859328 Dopamine has been widely used in the past for improving renal function but was abandoned due to lack of evidence and various potential serious side effects. In the new Heart Failure Guidelines 2013 of the AHA.pdf there is an interesting note in the section hospitalized patients with heart failure: low-dose dopamine infusion may be considered in addition to loop diuretic therapy to improve diuresis and better improve renal function. The level of evidence is IIb/B which means that efficacy is less well established and that there is greater conflicting evidence from trials. Indeed, when looking at the cited articles more questions than answer remain... but see yourself.
Giamouzis G, et al. .J Card Fail. 2010 Dec;16(12):922-30 Elkayam U, et al. Circulation. 2008 Jan 15;117(2):200-205 |
Search
|



 RSS Feed
RSS Feed


