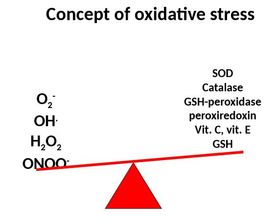
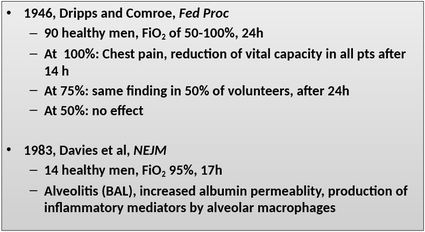
 Oxygen is life! There is no doubt, a lack of oxygen is no good and can have lethal consequences in humans. The mitochondrial reduction of oxygen sustains life in aerobic organisms and therefore makes oxygen to one of our most used medications in critical care. The Free Radical Theory But as important oxygen is to help sustain life, it's excess (hyperoxia) has proven to be actually harmful itself. This post provides a brief insight into this topic including some of the important references. Oxygen toxicity is attributed to the free radical theory in which the partial reduction of oxygen forms the superoxide anion radical. Superoxide is a toxic free radical with an unpaired valence shell electron in the outer orbital and is one of several free radicals known. These reactive oxygen species are formed primarily in the mitochondria, but also by neutrophils and endothelial cells and by the conversion of xanthines. The anti-oxidant defence mechanisms on the other hand consist of several enzymes and small molecules (Vit. A, C and E, Coenzyme Q etc.). An imbalance of these reactions leads to oxidative stress resulting in tissue damage. Lung Toxicity Already back in 1899 J. Lorrain Smith et al. noticed the toxic effect of high oxygen concentrations. It had been observed that "oxygen at a tension of over 100% of an atmosphere produced pneumonia in the normal animal". In their conclusion (at that time called 'General Résumé') they state: "Oxygen which at the tension of the atmosphere stimulates the lung cells to active absorption, at a higher tension acts as an irritant, or pathological stimulant, and produces inflammation". The pulmonary toxicity of oxygen is therefore sometimes still called the 'Lorrain Smith Effect'. Over the years several investigations have shown the toxic properties of oxygen in mice, primates and also humans. Gerald Nash et al. finally brought this topic to the level of critical care with an article in the NEJM in 1967. He performed autopsies in 70 patients that had been on mechanical ventilation with high inspired fractions of oxygen. He was able to show that lung damage correlated with the level of FiO2 applied. He also noticed that early changes were mainly oedematous and exudative while the late phase was characterised by fibro-proliferative changes. Other Implications of Hyperoxia
Haque W et al. J Am Coll Cardiol. 1996 Feb;27(2):353-7.
Latest NewsAnother interesting input on this topic comes from Girardi and his team who have published an interesting randomised clinical trial comparing the treatment of ICU patients with either oxygen in a conventional manner or according to a restrictive protocol. In this single-centre study his team have recruited 480 patients with an expected intensive care unit length of stay of 72 hours or longer. In this interdisciplinary ICU the patients were then randomly assigned to receive oxygen therapy to maintain a Pao2 between 70 and 100 mm Hg (or Spo2 between 94% and 98%, 'conservative group') or, according to standard ICU practice, to allow Pao2 values up to 150 mm Hg (or Spo2 between 97% and 100%, 'conventional control group'). Interestingly, the conservative protocol was associated with an absolute risk reduction for intensive care unit mortality of 8.6% compared with that for patients treated with conventional therapy. The patient populations were comparable, but still there are some limitations to this study. One of them is that the trial was terminated early due to an earthquake in the region of the hospital. Nevertheless, these findings earn respect. Take Home Messages
- Oxygen is definitely toxic at high concentrations! - Oxygen toxicity does not only depend on the concentration of oxygen applied it also depends on the duration of exposure! What should you do? - Oxygen is a drug, you need to dose accordingly! - Keep the FiO2 <0.6 whenever possible... and always at the lowest acceptable - Current recommendation is to achieve paO2 values of 60-80mmHg (ARDSnet.org) And Remember: Never withhold oxygen in an emergency setting, especially when the patient needs it! |
Search
|

 RSS Feed
RSS Feed


