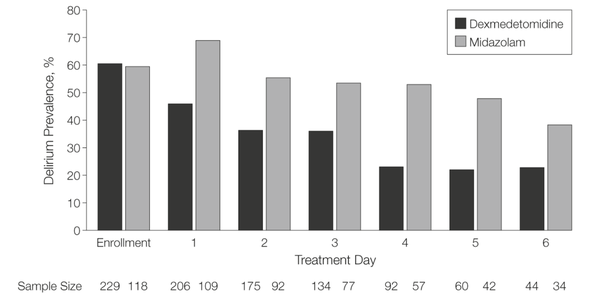
 When the FDA approved dexmedetomidine (DEX) in 1999, intensive care medicine had a novel and highly promising drug at its disposal. Compared to clonidine, dexmedetomidine is an 8 times more selective, central alpha 2 agonist, which binds to all 3 subtypes of the receptor. The properties of this substance were auspicious, among them: sedation, analgesia, neuroprotective effects and a lack of respiratory depression. - Sedation decreases sympathetic activity, aggression and leads to a non-REM-like state, which of all sedatives comes closest to natural sleep. Cognitive functions are maintained, and patients usually remain arousable. - Dexmedetomidine has a particular analgesic effect via modulation in the region of the posterior horn of the spinal cord. This has shown to reduce the use of opiates. - By reducing cerebral catecholamines, dexmedetomidine exerts a neuroprotective effect. - Interestingly, sedation with dexmedetomidine is not associated with significant respiratory depression. These properties pointed to a wide range of applications in the intensive care unit: - Sedation in patients with non-invasive ventilation - Weaning of invasively ventilated patients - Agitated delirium - Treatment of various withdrawal syndromes - Fiberoptic awake intubation in theatre conditions Dexmedetomidine comes with its side effects, though. Most commonly bradycardia and hypotension are observed, making second and third-degree heart block a contraindication. Also, nausea and a dry mouth might be seen. Interestingly, prolonged use might be associated with some extent of discontinuation syndrome similar to clonidine. This involves hypertension, tachycardia, nervousness etc. What Evidence Do We Have So Far? |
Search
|









 RSS Feed
RSS Feed


