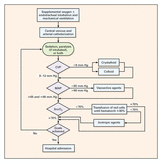
 In 2001 Rivers at al. published their remarkable Study on Early Goal Directed Therapy (EGDT) in Severe Sepsis and Septic Shock. The results of this single centre study were impressive, as this intervention significantly reduced mortality when applied within 6 hours after admission. Despite the implication of these principles into current guidelines many elements of the EGDT are being discussed quite controversially since. Especially goal criteria like central venous pressure (CVP) and mean arterial pressure (MAP) have been increasingly questioned and other elements like the trigger to transfuse red blood cell concentrates and the usage of dobutamine are also on going subjects of discussions. In order to answer some of these questions three big trials were started: - The ProCESS trial (Protocolised Care for Early Septic Shock) Out now! - The ARISE study (Australian Resuscitation in Sepsis Evalutation) Out Now! - The ProMISe study (Protocolised Management of Sepsis, GB) Coming up! ProCESS trial: In this multi-centre study a total of 1531 patients were included and randomised to one of the three following treatment groups: protocol-based EGDT (according to the criteria used in the River's study 2001), protocol based standard treatment (using 6-hours resuscitation instructions with no need for placement of a central venous catheter, administration of inotropes to maintain a certain systolic BP and blood transfusions if haemoglobin level was below 75g/L) or usual care (according to the physician's own assessment). Primary endpoint was 60-day in-hospital mortality. Interestingly the use vasopressors and red blood cell concentrates varied between the groups significantly but there was no difference in mortality by 60 days, 90 days or 1 year. Also the need for organ support showed no difference among the three groups. The ARISe trial is also a multi-centre study where patients with early septic shock were randomly assigned to either receive EGDT or usual care. A total of 1600 patients were included and primary outcome was all-cause mortality within 90 days after randomisation. Also here the two groups showed significant differences in regards of treatment modalities: the EGDT group received larger amounts of IV fluids, were more likely to receive vasopressors, got more re blood cell transfusions and were more likely to be treated with dobutamine. These differences are similar to the ones observed in the ProCESS trial. Again though, there was no difference in all-cause mortality at 90 days. So two high quality trials showed no difference between EGDT and 'usual' care. Does this mean that EGDT is of no use? The answer is: Not at all! Taking into account that Rivers published his study in 2001 many physicians involved in critical care are now 'children of this revolution'. Most intensivists nowadays are trained and aware of the importance of an EARLY treatment. The core message of River's study remains the same: Early treatment is important! It is remarkable though that a protocolled approach is non-superior to the clinical judgement of the treating physician. And I personally feel happy about the fact that protocols don't replace clinical judgement and an individual approach at all. Summarised: The key for a successful sepsis treatment remains the same: Treat EARLY (especially with the administration of antibiotics). And, maybe even more importantly: Simple protocols can be helpful, but DO NOT replace an individual, clinical assessment of the patient with severe sepsis or septic shock. We are looking forward for the results of the third trial pending - ProMISe The Process Investigators, N Engl J Med 2014 The ARISe Investigators, N Engl J Med 2014 Rivers et al. N Engl J Med 2001; 345:1368-1377 Read also: Guidelines Surviving Sepsis Campaign 2008, Dellinger et al. Crit Care Med 2008; 36:296 Guidelines Surviving Sepsis Campaign 2012, Dellinger et al. Crit Care Med 2013; 41:580 BIJC Review: Restricitve Transfusion Threshold is OK in Sepsis - The TRISS Trial Comments are closed.
|
Search
|

 RSS Feed
RSS Feed


